Label: HYDROXYZINE HYDROCHLORIDE injection, solution
- NDC Code(s): 0517-4201-25, 0517-5601-25, 0517-5602-25, 0517-5610-25
- Packager: American Regent, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 6, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
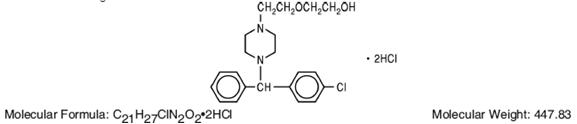
Hydroxyzine hydrochloride has the chemical name of (±)-2-[2-[4-(p-Chloro-α-phenylbenzyl)-1-piperazinyl]ethoxy]ethanol dihydrochloride and occurs as a white, odorless powder which is very soluble in water.
It has the following structural formula:
Hydroxyzine Hydrochloride Injection, USP is a sterile aqueous solution intended for intramuscular administration.
Each mL contains: Hydroxyzine HCl 25 mg or 50 mg, Benzyl Alcohol 0.9%, and Water for Injection q.s. pH adjusted with Sodium Hydroxide and/or Hydrochloric Acid.
-
CLINICAL PHARMACOLOGY
Hydroxyzine hydrochloride is unrelated chemically to phenothiazine, reserpine, and meprobamate. Hydroxyzine has demonstrated its clinical effectiveness in the chemotherapeutic aspect of the total management of neuroses and emotional disturbances manifested by anxiety, tension, agitation, apprehension or confusion.
Hydroxyzine has been shown clinically to be a rapid-acting true ataraxic with a wide margin of safety. It induces a calming effect in anxious, tense, psychoneurotic adults and also in anxious, hyperkinetic children without impairing mental alertness. It is not a cortical depressant, but its action may be due to a suppression of activity in certain key regions of the subcortical area of the central nervous system.
Primary skeletal muscle relaxation has been demonstrated experimentally.
Hydroxyzine has been shown experimentally to have antispasmodic properties, apparently mediated through interference with the mechanism that responds to spasmogenic agents such as serotonin, acetylcholine, and histamine.
Antihistaminic effects have been demonstrated experimentally and confirmed clinically.
An antiemetic effect, both by the apomorphine test and the veriloid test, has been demonstrated. Pharmacological and clinical studies indicate that hydroxyzine in therapeutic dosage does not increase gastric secretion or acidity and in most cases provides mild antisecretory benefits.
-
INDICATIONS AND USAGE
The total management of anxiety, tension, and psychomotor agitation in conditions of emotional stress requires in most instances a combined approach of psychotherapy and chemotherapy. Hydroxyzine has been found to be particularly useful for this latter phase of therapy in its ability to render the disturbed patient more amenable to psychotherapy in long term treatment of the psychoneurotic and psychotic, although it should not be used as the sole treatment of psychosis or of clearly demonstrated cases of depression.
Hydroxyzine is also useful in alleviating the manifestations of anxiety and tension as in the preparation for dental procedures and in acute emotional problems. It has also been recommended for the management of anxiety associated with organic disturbances and as adjunctive therapy in alcoholism and allergic conditions with strong emotional overlay, such as in asthma, chronic urticaria, and pruritus.
Hydroxyzine hydrochloride intramuscular solution is useful in treating the following types of patients when intramuscular administration is indicated:
1. The acutely disturbed or hysterical patient.
2. The acute or chronic alcoholic with anxiety withdrawal symptoms or delirium tremens.
3. As pre-and postoperative and pre- and postpartum adjunctive medication to permit reduction in narcotic dosage, allay anxiety and control emesis.
Hydroxyzine hydrochloride has also demonstrated effectiveness in controlling nausea and vomiting, excluding nausea and vomiting of pregnancy. (See CONTRAINDICATIONS).
In prepartum states, the reduction in narcotic requirement effected by hydroxyzine is of particular benefit to both mother and neonate.
Hydroxyzine benefits the cardiac patient by its ability to allay the associated anxiety and apprehension attendant to certain types of heart disease. Hydroxyzine is not known to interfere with the action of digitalis in any way and may be used concurrently with this agent.
The effectiveness of hydroxyzine in long term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should reassess periodically the usefulness of the drug for the individual patient.
-
CONTRAINDICATIONS
Hydroxyzine hydrochloride intramuscular solution is intended only for intramuscular administration and should not, under any circumstances, be injected subcutaneously, intra-arterially or intravenously.
Hydroxyzine is contraindicated in patients with a prolonged QT interval.This drug is contraindicated for patients who have shown a previous hypersensitivity to it.
Hydroxyzine, when administered to the pregnant mouse, rat, and rabbit, induced fetal abnormalities in the rat at doses substantially above the human therapeutic range. Clinical data in human beings are inadequate to establish safety in early pregnancy. Until such data are available, hydroxyzine is contraindicated in early pregnancy.
- WARNINGS
-
PRECAUTIONS
THE POTENTIATING ACTION OF HYDROXYZINE MUST BE CONSIDERED WHEN THE DRUG IS USED IN CONJUNCTION WITH CENTRAL NERVOUS SYSTEM DEPRESSANTS SUCH AS NARCOTICS, BARBITURATES AND ALCOHOL. Rarely, cardiac arrests and death have been reported in association with the combined use of hydroxyzine hydrochloride intramuscularly and other CNS depressants. Therefore, when central nervous system depressants are administered concomitantly with hydroxyzine their dosage should be reduced up to 50 percent. The efficacy of hydroxyzine as adjunctive pre- and postoperative sedative medication has also been well established, especially as regards its ability to allay anxiety, control emesis, and reduce the amount of narcotic required.
HYDROXYZINE MAY POTENTIATE NARCOTICS AND BARBITURATES, so their use in preanesthetic adjunctive therapy should be modified on an individual basis. Atropine and other belladonna alkaloids are not affected by the drug.
When hydroxyzine is used preoperatively or prepartum, narcotic requirements may be reduced as much as 50 percent. Thus, when 50 mg of hydroxyzine hydrochloride intramuscular solution is employed, meperidine dosage may be reduced from 100 mg to 50 mg. The administration of meperidine may result in severe hypotension in the postoperative patient or any individual whose ability to maintain blood pressure has been compromised by a depleted blood volume. Meperidine should be used with great caution and in reduced dosage in patients who are receiving other pre- and/or postoperative medications and in whom there is a risk of respiratory depression, hypotension, and profound sedation or coma occurring. Before using any medications concomitant with hydroxyzine, the manufacturer"s prescribing information should be read carefully.
Since drowsiness may occur with the use of this drug, patients should be warned of this possibility and cautioned against driving a car or operating dangerous machinery while taking this drug.
As with all intramuscular preparations, hydroxyzine hydrochloride intramuscular solution should be injected well within the body of a relatively large muscle. Inadvertent subcutaneous injection may result in significant tissue damage.
Adults
The preferred site is the upper outer quadrant of the buttock, (i.e., the gluteus maximus), or the mid-lateral thigh.
Children
It is recommended that intramuscular injections be given preferably in the mid-lateral muscles of the thigh. In infants and small children the periphery of the upper outer quadrant of the gluteal region should be used only when necessary, such as in burn patients, in order to minimize the possibility of damage to the sciatic nerve.
The deltoid area should be used only if well developed such as in certain adults and older children, and then only with caution to avoid radial nerve injury. Intramuscular injections should not be made into the lower and mid-third of the upper arm. As with all intramuscular injections, aspiration is necessary to help avoid inadvertent injection into a blood vessel.
Geriatric Use
A determination has not been made whether controlled clinical studies of hydroxyzine hydrochloride included sufficient numbers of subjects aged 65 and over to define a difference in response from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
The extent of renal excretion of hydroxyzine hydrochloride has not been determined. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selections.
Sedating drugs may cause confusion and over-sedation in the elderly; elderly patients generally should be started on low doses of hydroxyzine hydrochloride and observed closely.
CASES OF QT PROLONGATION AND TORSADE DE POINTES HAVE BEEN REPORTED DURING POST-MARKETING USE OF HYDROXYZINE. The majority of reports occurred in patients with other risk factors for QT prolongation or Torsade de Pointes (pre-existing heart disease, electrolyte imbalances or concomitant arrhythmogenic drug use). Therefore, hydroxyzine should be used with caution in patients with risk factors for QT prolongation, congenital long QT syndrome, a family history of long QT syndrome, other conditions that predispose to QT prolongation and ventricular arrhythmia, as well as recent myocardial infarction, uncompensated heart failure, and bradyarrhythmias.Caution is recommended during the concomitant use of drugs known to prolong the QT interval. These include Class 1A (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmics, certain antipsychotics (e.g., ziprasidone, iloperidone, clozapine, quetiapine, chlorpromazine), certain antidepressants (e.g., citalopram, fluoxetine), certain antibiotics (e.g., azithromycin, erythromycin, clarithromycin, gatifloxacin, moxifloxacin); and others (e.g., pentamidine, methadone, ondansetron, droperidol).
Acute Generalized Exanthematous Pustulosis (AGEP)
Hydroxyzine may rarely cause acute generalized exanthematous pustulosis (AGEP), a serious skin reaction characterized by fever and numerous small, superficial, nonfollicular, sterile pustules, arising within large areas of edematous erythema. Inform patients about the signs of AGEP, and discontinue hydroxyzine at the first appearance of a skin rash, worsening of pre-existing skin reactions which hydroxyzine may be used to treat, or any other sign of hypersensitivity. If signs or symptoms suggest AGEP, use of hydroxyzine should not be resumed and alternative therapy should be considered. Avoid cetirizine or levocetirizine in patients who have experienced AGEP or other hypersensitivity reactions with hydroxyzine, due to the risk of cross-sensitivity.
-
ADVERSE REACTIONS
Therapeutic doses of hydroxyzine seldom produce impairment of mental alertness. However, drowsiness may occur; if so, it is usually transitory and may disappear in a few days of continued therapy or upon reduction of the dose. Dryness of the mouth may be encountered at higher doses. Extensive clinical use has substantiated the absence of toxic effects on the liver or bone marrow when administered in the recommended doses for over four years of uninterrupted therapy. The absence of adverse effects has been further demonstrated in experimental studies in which excessively high doses were administered.
Involuntary motor activity, including rare instances of tremor and convulsions, has been reported, usually with doses considerably higher than those recommended. Continuous therapy with over one gram per day has been employed in some patients without these effects having been encountered. Hydroxyzine hydrochloride is associated with Acute Generalized Exanthematous Pustulosis (AGEP) in post marketing reports.
-
DOSAGE AND ADMINISTRATION
The recommended dosages for hydroxyzine hydrochloride intramuscular solution are:
For adult psychiatric and emotional emergencies, including acute alcoholism.
Intramuscular: 50 to100 mg stat., and every 4 to 6 hours as needed. Nausea and vomiting excluding nausea and vomiting of pregnancy. Adults: 25 to 100 mg intramuscularly
Children: 0.5 mg/lb body weight intramuscularly
Pre- and postoperative adjunctive medication. Adults: 25 to 100 mg intramuscularly
Children: 0.5 mg/lb body weight intramuscularly
Pre- and postpartum adjunctive therapy. 25 to 100 mg intramuscularly As with all potent medications, the dosage should be adjusted according to the patient's response to therapy.
FOR ADDITIONAL INFORMATION ON THE ADMINISTRATION AND SITE OF SELECTION SEE PRECAUTIONS SECTION. NOTE: Hydroxyzine hydrochloride intramuscular solution may be administered without further dilution.
Patients may be started on intramuscular therapy when indicated. They should be maintained on oral therapy whenever this route is practicable.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
-
HOW SUPPLIED
Product No. Strength Size NDC-0517-4201-25 25 mg/mL 1 mL Single Dose Vial Boxes of 25 NDC-0517-5601-25 50 mg/mL 1 mL Single Dose Vial Boxes of 25 NDC-0517-5602-25 50 mg/mL 2 mL Single Dose Vial Boxes of 25 NDC-0517-5610-25 50 mg/mL 10 mL Multiple Dose Vial Boxes of 25 Storage Condition: Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature). Protect from light. Discard unused portion of the single dose vial.
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967IN4201
Rev. 10/16 -
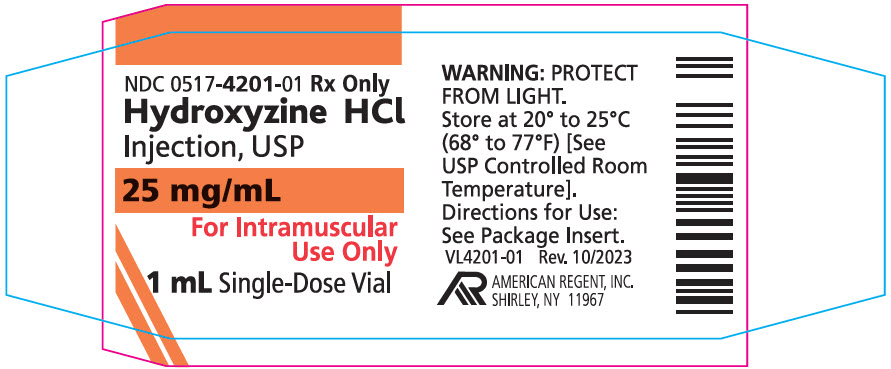
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 1 mL (25 mg)
PRINCIPAL DISPLAY PANEL – 1 mL (25 mg) Container
NDC 0517-4201-25
HYDROXYZINE HCl
INJECTION, USP
25 mg/mL
1 mL SINGLE DOSE VIALFOR IM USE ONLY
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967

PRINCIPAL DISPLAY PANEL – 1 mL (25 mg) Carton
HYDROXYZINE HCl
INJECTION, USP
25 mg/mLNDC 0517-4201-25
25 x 1 mL SINGLE DOSE VIALS
FOR INTRAMUSCULAR USE ONLY
Rx Only
Each mL contains: Hydroxyzine HCl 25 mg, Benzyl Alcohol 0.9%, Water for Injection q.s. pH adjusted with Sodium Hydroxide and/or Hydrochloric Acid.
WARNING: PROTECT FROM LIGHT. DISCARD UNUSED PORTION.
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) (See USP Controlled Room Temperature).
Directions for Use: See Package Insert.AMERICAN
REGENT, INC.
SHIRLEY, NY 11967Rev. 11/05

-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 1 mL (50 mg)
PRINCIPAL DISPLAY PANEL – 1 mL (50 mg) Container
NDC 0517-5601-25HYDROXYZINE HCl
INJECTION, USP
50 mg/mL1 mL SINGLE DOSE VIAL
FOR IM USE ONLY
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967
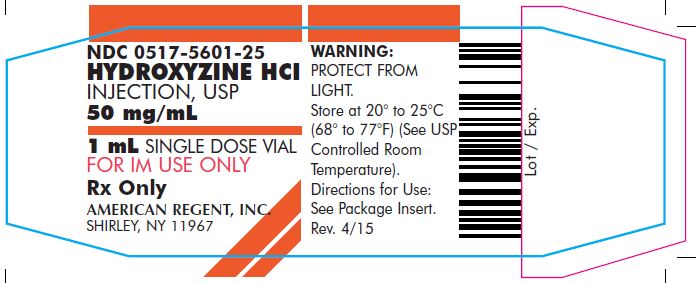
PRINCIPAL DISPLAY PANEL – 1 mL (50 mg) Carton
HYDROXYZINE HCl
INJECTION, USP
50 mg/mLNDC 0517-5601-25
25 x 1 mL SINGLE DOSE VIALS
FOR INTRAMUSCULAR USE ONLY
Rx Only
Each mL contains: Hydroxyzine HCl 50 mg, Benzyl Alcohol 0.9%, Water for Injection q.s. pH adjusted with Sodium Hydroxide and/or Hydrochloric Acid.
WARNING: PROTECT FROM LIGHT. DISCARD UNUSED PORTION.
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) (See USP Controlled Room Temperature).
Directions for Use: See Package Insert.AMERICAN
REGENT, INC.
SHIRLEY, NY 11967Rev.11/05

-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 2 mL (50 mg)
PRINCIPAL DISPLAY PANEL – 2 mL (50 mg) Container
NDC 0517-5602-25HYDROXYZINE HCl
INJECTION, USP
100 mg/2 mL (50 mg/mL)2 mL SINGLE DOSE VIAL
FOR IM USE ONLY
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967
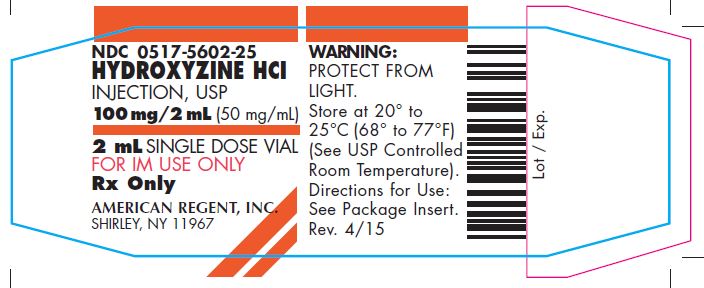
PRINCIPAL DISPLAY PANEL – 2 mL (50 mg) Carton
HYDROXYZINE HCl
INJECTION, USP
100 mg/2 mL (50 mg/mL)NDC 0517-5602-25
25 x 2 mL SINGLE DOSE VIALS
FOR INTRAMUSCULAR USE ONLY
Rx Only
Each mL contains: Hydroxyzine HCl 50 mg, Benzyl Alcohol 0.9%, Water for Injection q.s. pH adjusted with Sodium Hydroxide and/or Hydrochloric Acid.
WARNING: PROTECT FROM LIGHT. DISCARD UNUSED PORTION.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature).
Directions for Use: See Package Insert.AMERICAN
REGENT, INC.
SHIRLEY, NY 11967Rev. 11/05

- Serialization Label - 4201-25
- Serialization Label - 5601-25
- Serialization Label - 5602-25
-
INGREDIENTS AND APPEARANCE
HYDROXYZINE HYDROCHLORIDE
hydroxyzine hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0517-4201 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROXYZINE DIHYDROCHLORIDE (UNII: 76755771U3) (HYDROXYZINE - UNII:30S50YM8OG) HYDROXYZINE DIHYDROCHLORIDE 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0517-4201-25 25 in 1 TRAY 09/30/1990 1 1 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA087408 09/30/1990 HYDROXYZINE HYDROCHLORIDE
hydroxyzine hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0517-5601 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROXYZINE DIHYDROCHLORIDE (UNII: 76755771U3) (HYDROXYZINE - UNII:30S50YM8OG) HYDROXYZINE DIHYDROCHLORIDE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0517-5601-25 25 in 1 TRAY 09/30/1990 1 1 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA087408 09/30/1990 HYDROXYZINE HYDROCHLORIDE
hydroxyzine hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0517-5602 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROXYZINE DIHYDROCHLORIDE (UNII: 76755771U3) (HYDROXYZINE - UNII:30S50YM8OG) HYDROXYZINE DIHYDROCHLORIDE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0517-5602-25 25 in 1 TRAY 09/30/1990 1 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA087408 09/30/1990 HYDROXYZINE HYDROCHLORIDE
hydroxyzine hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0517-5610 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROXYZINE DIHYDROCHLORIDE (UNII: 76755771U3) (HYDROXYZINE - UNII:30S50YM8OG) HYDROXYZINE DIHYDROCHLORIDE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0517-5610-25 25 in 1 TRAY 09/30/1990 11/01/2016 1 10 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA087408 09/30/1990 11/01/2016 Labeler - American Regent, Inc. (002033710) Establishment Name Address ID/FEI Business Operations American Regent, Inc. 002033710 analysis(0517-4201, 0517-5601, 0517-5602, 0517-5610) , manufacture(0517-4201, 0517-5601, 0517-5602, 0517-5610)