Label: VETRISHIELD MAX- ivermectin/pyrantel pamoate/praziquantel tablet, chewable
- NDC Code(s): 13985-952-12, 13985-971-25, 13985-972-50, 13985-973-10
- Packager: VetOne
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated October 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- GENERAL PRECAUTIONS
- DESCRIPTION:
-
INDICATIONS:
For use in dogs to prevent canine heartworm disease by eliminating the tissue stage of heartworm larvae (Dirofilaria immitis) for a month (30 days) after infection and for the treatment and control of roundworms (Toxocara canis, Toxascaris leonina), hookworms (Ancylostoma caninum, Uncinaria stenocephala, Ancylostoma braziliense), and tapeworms (Dipylidium caninum, Taenia pisiformis).
-
DOSAGE:
VetriShield Max (ivermectin/pyrantel pamoate/praziquantel) Chewable Tablets should be administered orally at monthly intervals at the recommended minimum dose level of 6 mcg of ivermectin per kilogram (2.72 mcg/lb), 5 mg of pyrantel (as pamoate salt) per kg (2.27 mg/lb) and 5 mg of praziquantel per kg (2.27 mg/lb) of body weight, as follows:
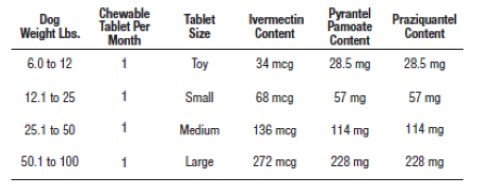
VetriShield Max Chewable Tablets are recommended for dogs 8 weeks of age and older. For dogs over 100 lbs, use the appropriate combination of these Chewable Tablets.
ADMINISTRATION: Remove only one tablet at a time from the foil-backed blister card. Return the remaining tablets to their box to protect the product from light. VetriShield Max Chewable Tablets can be offered to the dog by hand or be added intact to a small amount of dog food. Care should be taken that the dog consumes the complete dose, and the dog should be observed for a few minutes after administration to ensure that part of the dose is not lost or rejected. If it is suspected that any of the dose has been lost, redosing is recommended.
VetriShield Max Chewable Tablets should be given at monthly intervals during the period of the year when mosquitoes (vectors), potentially carrying infective heartworm larvae, are active. The initial dose must be given within a month (30 days) after the dog's first exposure to mosquitoes. The final dose must be given within a month (30 days) after the dog's last exposure to mosquitoes.
When replacing another heartworm preventive product in a heartworm disease prevention program, the first dose of VetriShield Max Chewable Tablets must be given within a month (30 days) of the last dose of the former medication. A heartworm test should be performed prior to switching heartworm preventive products.
If the interval between doses exceeds a month (30 days), the efficacy of ivermectin can be reduced. Therefore, for optimal performance, the tablet must be given once a month on or about the same day of the month. If treatment is delayed, whether by a few days or many, immediate treatment with VetriShield Max Chewable Tablets and resumption of the recommended dosing regimen will minimize the opportunity for the development of adult heartworms.
-
WARNING:
For use in dogs only. Keep this and all drugs out of reach of children. In safety studies, testicular hypoplasia was observed in some dogs receiving 3 and 5 times the maximum recommended dose monthly for 6 months (see Animal Safety).
In case of ingestion by humans, clients should be advised to contact a physician immediately. Physicians may contact a Poison Control Center for advice concerning cases of ingestion by humans.
For a copy of the Material Safety Data Sheet (MSDS), or to report adverse reactions, call 1-800-338-3659.
PRECAUTIONS: Use with caution in sick, debilitated, or underweight animals and dogs weighing less than 10 lbs (see Animal Safety). The safe use of this drug has not been evaluated in pregnant or lactating bitches.
All dogs should be tested for existing heartworm infection before starting treatment with VetriShield Max™ Chewable Tablets, which are not effective against adult D. immitis. Infected dogs should be treated to remove adult heartworms and microfilariae before initiating a heartworm prevention program.
While some microfilariae may be killed by the ivermectin in VetriShield Max™ Chewable Tablets at the recommended dose level, VetriShield Max™ Chewable Tablets are not effective for microfilariae clearance. A mild hypersensitivity-type reaction, presumably due to dead or dying microfilariae and particularly involving a transient diarrhea, has been observed in clinical trials with ivermectin alone after treatment of some dogs that have circulating microfilariae.
-
ADVERSE REACTIONS:
Self-limiting adverse reactions including lethargy, limpness, salivation, shaking, diarrhea, decreased appetite, licking lips, and belching were reported between 20 minutes and 72 hours following treatment in a field study with VetriShield Max™ Chewable Tablets.
In clinical field trials with ivermectin/pyrantel pamoate, vomiting or diarrhea within 24 hours of dosing was rarely observed (1.1% of administered doses). The following adverse reactions have been reported following the use of ivermectin: depression/lethargy, vomiting, anorexia, diarrhea, mydriasis, ataxia, staggering, convulsions and hypersalivation.
-
EFFECTIVENESS:
VetriShield Max™ Chewable Tablets, given orally using the recommended dose and regimen, are effective against the tissue larval stage of D. immitis for a month (30 days) after infection and, as a result, prevent the development of the adult stage. VetriShield Max™ Chewable Tablets are also effective against canine roundworms (T. canis, T. leonina), hookworms (A. caninum, U. stenocephala, A. braziliense), and tapeworms (T. pisiformis, D. caninum).
A total of 61 dogs and puppies with naturally acquired or experimental parasite infections treated with VetriShield Max™ Chewable Tablets were enrolled in 7 well-controlled laboratory studies to establish effectiveness. These studies included dogs of various sizes, ages and breeds. Data from these studies demonstrated VetriShield Max™ Chewable Tablets are safe and effective for the removal of the parasite species indicated on the label when used as directed.
ANIMAL SAFETY: Studies with ivermectin indicate that certain dogs of the Collie breed are more sensitive to the effects of ivermectin administered at elevated dose levels (more than 16 times the target use level of 6 mcg/kg) than dogs of other breeds. At elevated doses, sensitive dogs showed adverse reactions which included mydriasis, depression, ataxia, tremors, drooling, paresis, recumbency, excitability, stupor, coma and death. No signs of toxicity were seen at 10 times the recommended dose (27.2 mcg/lb) in sensitive Collies. Results of these studies and bioequivalence studies support the safety of ivermectin products in dogs, including Collies, when used as recommended by the label.
In a target animal safety study, VetriShield Max™ Chewable Tablets were administered to 8-week old Beagle puppies at one, three and five times the maximum recommended dose of 12.5 mcg/kg ivermectin, 10.47 mg/kg pyrantel and 10.47 mg/kg praziquantel. The dogs were treated every 30 days for 6 months. Vomiting within 6 hours of dosing and soft or watery feces within 24 hours of dosing were observed. Other observations during the study were: anogenital swelling, lethargy, head movements, shallow, audible or difficult breathing, and salivation. One dog in the 5X group had tremors and decreased activity. All these signs were transient. No treatment was required. Pathology showed testicular hypoplasia in the 3 and 5X groups (see WARNINGS).
In a laboratory safety study, 12-week-old Beagle puppies receiving 3 and 5 times the recommended dose once weekly for 13 weeks demonstrated a dose-related decrease in testicular maturation compared to controls. In this study, all treated puppies had significantly higher cholesterol levels compared to untreated controls.
In a reproductive safety study, adult males were treated at 37.5 mcg/kg ivermectin, 31.4 mg/kg pyrantel and 31.4 mg/kg praziquantel every 14 days during two full spermatogenic cycles (112 days). The quality of semen and reproductive health were not affected by treatment. Treatment-related vomiting and soft feces were reported during this study.
In a study of the effectiveness of VetriShield Max™ Chewable Tablets for treatment of T. canis, one 8.1 lb, 72-day-old puppy died 6 days after administration of VetriShield Max™ Chewable Tablets at the label dose. This puppy and many of the other puppies in the study had high worm burdens and were reported to have diarrhea, sometimes bloody, frequently before and after treatment with VetriShield Max™ Chewable Tablets or the placebo. Dehydration and signs of anemia (pale mucous membranes) were the only abnormal gross necropsy findings observed. No definitive cause of death was determined. In a 90-day field study, the most serious ADEs (lethargy, limpness, and salivation) were seen in dogs weighing less than 10 lbs (see PRECAUTIONS).
- STORAGE INFORMATION:
- HOW SUPPLIED:
- SPL UNCLASSIFIED SECTION
- Toy 6.0-12.0 lbs
- Small 12.1-25.0 lbs
- Medium 26.0-50.0 lbs
- Large 51.0-100.0 lbs
-
INGREDIENTS AND APPEARANCE
VETRISHIELD MAX
ivermectin/pyrantel pamoate/praziquantel tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:13985-952 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IVERMECTIN (UNII: 8883YP2R6D) (IVERMECTIN - UNII:8883YP2R6D) IVERMECTIN 34 ug PYRANTEL PAMOATE (UNII: 81BK194Z5M) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL 28.5 mg PRAZIQUANTEL (UNII: 6490C9U457) (PRAZIQUANTEL - UNII:6490C9U457) PRAZIQUANTEL 28.5 mg Product Characteristics Color brown Score no score Shape ROUND Size 9mm Flavor LIVER Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13985-952-12 1 in 1 CARTON 1 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141257 10/12/2023 VETRISHIELD MAX
ivermectin/pyrantel pamoate/praziquantel tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:13985-971 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IVERMECTIN (UNII: 8883YP2R6D) (IVERMECTIN - UNII:8883YP2R6D) IVERMECTIN 68 ug PYRANTEL PAMOATE (UNII: 81BK194Z5M) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL 57 mg PRAZIQUANTEL (UNII: 6490C9U457) (PRAZIQUANTEL - UNII:6490C9U457) PRAZIQUANTEL 57 mg Product Characteristics Color brown Score no score Shape ROUND Size 11mm Flavor LIVER Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13985-971-25 1 in 1 CARTON 1 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141257 10/12/2023 VETRISHIELD MAX
ivermectin/pyrantel pamoate/praziquantel tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:13985-972 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IVERMECTIN (UNII: 8883YP2R6D) (IVERMECTIN - UNII:8883YP2R6D) IVERMECTIN 136 ug PYRANTEL PAMOATE (UNII: 81BK194Z5M) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL 114 mg PRAZIQUANTEL (UNII: 6490C9U457) (PRAZIQUANTEL - UNII:6490C9U457) PRAZIQUANTEL 114 mg Product Characteristics Color brown Score no score Shape ROUND Size 15mm Flavor LIVER Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13985-972-50 1 in 1 CARTON 1 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141257 10/12/2023 VETRISHIELD MAX
ivermectin/pyrantel pamoate/praziquantel tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:13985-973 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IVERMECTIN (UNII: 8883YP2R6D) (IVERMECTIN - UNII:8883YP2R6D) IVERMECTIN 272 ug PYRANTEL PAMOATE (UNII: 81BK194Z5M) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL 228 mg PRAZIQUANTEL (UNII: 6490C9U457) (PRAZIQUANTEL - UNII:6490C9U457) PRAZIQUANTEL 228 mg Product Characteristics Color brown Score no score Shape ROUND Size 19mm Flavor LIVER Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13985-973-10 1 in 1 CARTON 1 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141257 10/12/2023 Labeler - VetOne (019926120)