Label: TORBUGESIC SA- butorphanol tartrate solution
- NDC Code(s): 54771-4531-1
- Packager: Zoetis Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated March 20, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CAUTION
-
DESCRIPTION
Butorphanol tartrate, is a synthetic, centrally acting, narcotic agonist-antagonist analgesic with potent antitussive activity. The results from laboratory and clinical studies suggest the existence of several distinct types of receptors that are responsible for the activity of opioid and opioid-like drugs. When activated, the μ(mu)-receptors are involved in analgesia, respiratory depression, miosis, physical dependence and feelings of well-being (euphoria). When activated, the κ(kappa)-receptors are involved in analgesia, as well as less intense (as compared to μ-receptors) miosis and respiratory depression. Butorphanol is considered to be a weak antagonist at the μ-receptor, but a strong agonist at the κ-receptor. Thus, butorphanol provides analgesia with a lower incidence and/or intensity of adverse reactions (e.g., miosis and respiratory depression) than traditional opioids.
Butorphanol tartrate is a member of the phenanthrene series. The chemical name is Morphinan-3, 14-diol, 17-(cyclobutylmethyl)-, (-)-, (S- (R*, R*))- 2,3- dihydroxybutanedioate (1:1) (salt). It is a white, crystalline, water soluble substance having a molecular weight of 477.55; its molecular formula is C21H29NO2• C4H6O6.
Chemical Structure
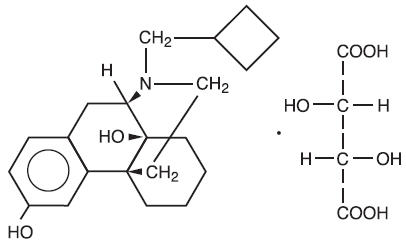
Each mL of TORBUGESIC-SA contains 2 mg butorphanol base (as butorphanol tartrate, USP); 3.3 mg citric acid, USP; 6.4 mg sodium citrate, USP; 4.7 mg sodium chloride; and 0.1 mg benzethonium chloride; q.s. with water for injection.
-
CLINICAL PHARMACOLOGY
Feline Pharmacology
The magnitude and duration of analgesic activity of butorphanol were studied in cats under controlled laboratory conditions using both a visceral pain model and a somatic pain model.1,2 Subcutaneous butorphanol dosages of 0.4 mg/kg produced analgesia significantly (p<0.05) greater than the placebo for up to two hours in the somatic pain model. At the label dose (0.4 mg/kg), cardiopulmonary depressant effects were minimal after treatment with butorphanol as demonstrated in cats.1,2
Clinical studies confirmed the analgesic effect of butorphanol administered subcutaneously in the cat. In field trials the overall analgesic effect was rated as satisfactory in approximately 75% of butorphanol treated cats. The duration of activity in cats responding to butorphanol ranged from 15 minutes to 8 hours. However, in 70% of responding cats the duration of activity was 3 to 6 hours following subcutaneous administration
Safety Studies in Cats
Daily subcutaneous injections of butorphanol in cats, beginning at a dosage of 2 mg/kg the first week and doubling each week to a final dosage of 16 mg/kg on the fourth week, resulted in no deaths. No evidence of toxicity was observed during the first three weeks of the experiment, other than pain on injection. During the fourth week, transient incoordination, salivation, or mild seizures were observed within the first hour in the cats following the 16 mg/kg dosage (40 times the recommended clinical dosage). No other clinical, serum chemistry, or gross necropsy evidence of drug toxicity was encountered in any of the cats.
In subacute safety studies, butorphanol was injected subcutaneously to each of six cats at dosages of 0 (saline), 0.4, 1.2 or 2.0 mg/kg, every six hours for six days and continued once daily for a total of 21 days. The only adverse clinical effect observed was pain on injection. Histopathologic changes indicative of minimal to slight irritation were noted at the injection sites in 3 of 6 cats in the low dose group, 4 of 6 cats in the middle dose group and 6 of 6 cats in the high dose group. Histopathologic changes of focal renal tubular dilation were noted in half of the cats in the high dose group.
- INDICATIONS
- WARNINGS
-
PRECAUTIONS
TORBUGESIC-SA, a potent analgesic, should be used with caution with other sedative or analgesic drugs as these are likely to produce additive effects.
Safety for use in pregnant female cats, breeding male cats or kittens less than 4 months of age has not been determined. Use of TORBUGESIC-SA can therefore not be recommended in these groups.
- ADVERSE REACTIONS
-
DOSAGE
The recommended dosage in cats is 0.4 mg of butorphanol per kilogram body weight (0.2 mg/lb) given by subcutaneous injection. This is equivalent to 1.0 mL of TORBUGESIC-SA per 10 lbs of body weight.
Pre-clinical model studies and clinical field trials in cats demonstrated that the analgesic effects of TORBUGESIC-SA are seen within 20 minutes and persist in the majority of responding cats for 3 to 6 hours following subcutaneous injection (see Feline Pharmacology). The dose may be repeated up to 4 times per day for up to 2 days.
Use contents within 4 months of first puncture.
- SUPPLY
- REFERENCES
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 10 mL Label
-
INGREDIENTS AND APPEARANCE
TORBUGESIC SA
butorphanol tartrate solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:54771-4531 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUTORPHANOL TARTRATE (UNII: 2L7I72RUHN) (BUTORPHANOL - UNII:QV897JC36D) BUTORPHANOL TARTRATE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 3.3 mg in 1 mL SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM CHLORIDE (UNII: 451W47IQ8X) BENZETHONIUM CHLORIDE (UNII: PH41D05744) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-4531-1 10 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141047 06/11/1985 Labeler - Zoetis Inc. (828851555) Establishment Name Address ID/FEI Business Operations Teva Czech Industries s.r.o 643896244 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Zoetis Manufacturing & Research Spain, S.L. 460052343 MANUFACTURE


