Label: MYCAMINE- micafungin sodium injection, powder, lyophilized, for solution
- NDC Code(s): 0469-3211-10, 0469-3250-10
- Packager: Astellas Pharma US, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 4, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MYCAMINE® safely and effectively. See full prescribing information for MYCAMINE®.
MYCAMINE® (micafungin for injection), for intravenous use
Initial U.S. Approval: 2005RECENT MAJOR CHANGES
INDICATIONS AND USAGE
MYCAMINE® is an echinocandin indicated in adult and pediatric patients for (1):
- •
- Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesses in adult and pediatric patients 4 months of age and older.
- •
- Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesses without meningoencephalitis and/or ocular dissemination in pediatric patients younger than 4 months of age.
- •
- Treatment of Esophageal Candidiasis in adult and pediatric patients 4 months of age and older.
- •
- Prophylaxis of Candida Infections in adult and pediatric patients 4 months of age and older undergoing Hematopoietic Stem Cell Transplantation (HSCT).
Limitations of Use
- •
- The safety and effectiveness of MYCAMINE have not been established for the treatment of candidemia with meningoencephalitis and/or ocular dissemination in pediatric patients younger than 4 months of age as a higher dose may be needed. (1, 2.3, 8.4)
- •
- MYCAMINE has not been adequately studied in patients with endocarditis, osteomyelitis or meningoencephalitis due to Candida. (1)
- •
- The efficacy of MYCAMINE against infections caused by fungi other than Candida has not been established. (1)
DOSAGE AND ADMINISTRATION
Recommended Dosage Administered by Indication, Weight and Age (2.1, 2.2, 2.3, 8.4)
Adult
Pediatric Patients
4 Months and Older
30 kg or lessPediatric Patients
4 Months and Older
greater than 30 kgPediatric Patients Younger than 4 Months of Age
Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesses
100 mg daily
2 mg/kg/day
(maximum 100 mg daily)See below
Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesses without Meningoencephalitis and/or Ocular Dissemination
See above
See above
4 mg/kg/day
Treatment of Esophageal Candidiasis
150 mg daily
3 mg/kg/day
2.5 mg/kg/day
(maximum
150 mg daily)Not approved
Prophylaxis of Candida Infections in HSCT Recipients
50 mg daily
1 mg/kg/day
(maximum 50 mg daily)Not approved
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
MYCAMINE is contraindicated in persons with known hypersensitivity to micafungin sodium, any component of MYCAMINE, or other echinocandins. (4)
WARNINGS AND PRECAUTIONS
- •
- Hypersensitivity Reactions: Anaphylaxis and anaphylactoid reactions (including shock) have been observed. Discontinue MYCAMINE and administer appropriate treatment. (5.1)
- •
- Hematological Effects: Isolated cases of acute intravascular hemolysis, hemolytic anemia and hemoglobinuria have been reported. Monitor rate of hemolysis. Discontinue if severe. (5.2)
- •
- Hepatic Effects: Abnormalities in liver tests; isolated cases of hepatic impairment, hepatitis, and hepatic failure have been observed. Monitor hepatic function. Discontinue if severe dysfunction occurs. (5.3)
- •
- Renal Effects: Elevations in BUN and creatinine; isolated cases of renal impairment or acute renal failure have been reported. Monitor renal function. (5.4)
- •
- Infusion and Injection Site Reactions can occur including rash, pruritus, facial swelling, and vasodilatation. Monitor infusion closely, slow infusion rate if necessary. (2.5, 5.5)
ADVERSE REACTIONS
- •
- Most common adverse reactions across adult and pediatric clinical trials for all indications include diarrhea, nausea, vomiting, abdominal pain, pyrexia, thrombocytopenia, neutropenia, and headache. (6.1)
- •
- In pediatric patients younger than 4 months of age, the following additional common adverse reactions were reported at an incidence rate of ≥15%: sepsis, acidosis, anemia, oxygen saturation decreased and hypokalemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Astellas Pharma US, Inc. at 1-800-727-7003 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Monitor for sirolimus, itraconazole or nifedipine toxicity, and dosage of sirolimus, itraconazole or nifedipine should be reduced, if necessary. (7)
USE IN SPECIFIC POPULATIONS
Pregnancy – Based on animal data, MYCAMINE may cause fetal harm. Advise pregnant women of the risk to the fetus. (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Adults
2.2 Dosage for Pediatric Patients 4 Months and Older
2.3 Dosage for Pediatric Patients Younger than 4 Months of Age
2.4 Directions for Reconstitution, Dilution, and Preparation
2.5 Infusion Volume and Duration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Hematological Effects
5.3 Hepatic Effects
5.4 Renal Effects
5.5 Infusion and Injection Site Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on MYCAMINE
7.2 Effect of MYCAMINE on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Use in Patients with Renal Impairment
8.7 Use in Patients with Hepatic Impairment
8.8 Race and Gender
9 DRUG ABUSE AND DEPENDENCE
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Treatment of Candidemia and Other Candida Infections in Adult and Pediatric Patients 4 Months of Age and Older
14.2 Treatment of Esophageal Candidiasis in Adult and Pediatric Patients 4 Months of Age and Older
14.3 Prophylaxis of Candida Infections in Hematopoietic Stem Cell Transplant Recipients
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
MYCAMINE® is indicated for:
• Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesses in adult and pediatric patients 4 months of age and older [see Clinical Studies (14.1) and Use in Specific Populations (8.4)].
• Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesses without meningoencephalitis and/or ocular dissemination in pediatric patients younger than 4 months of age [see Use in Specific Populations (8.4)].
• Treatment of Esophageal Candidiasis in adult and pediatric patients 4 months of age and older [see Clinical Studies (14.2)].
• Prophylaxis of Candida Infections in adult and pediatric patients 4 months of age and older undergoing hematopoietic stem cell transplantation [see Clinical Studies (14.3)].Limitations of Use
• The safety and effectiveness of MYCAMINE have not been established for the treatment of candidemia with meningoencephalitis and/or ocular dissemination in pediatric patients younger than 4 months of age as a higher dose may be needed [see Use in Specific Populations (8.4)].
• MYCAMINE has not been adequately studied in patients with endocarditis, osteomyelitis and meningoencephalitis due to Candida.
• The efficacy of MYCAMINE against infections caused by fungi other than Candida has not been established. -
2 DOSAGE AND ADMINISTRATION
2.1 Dosage for Adults
The recommended dosage for adult patients based on indications are shown in Table 1.
Table 1. MYCAMINE Dosage in Adult Patients - *
- In patients treated successfully for candidemia and other Candida infections, the mean duration of treatment was 15 days (range 10 to 47 days).
- †
- In patients treated successfully for esophageal candidiasis, the mean duration of treatment was 15 days (range 10 to 30 days).
- ‡
- In hematopoietic stem cell transplant (HSCT) recipients who experienced success of prophylactic therapy, the mean duration of prophylaxis was 19 days (range 6 to 51 days).
Indication
Recommended Reconstituted Dose Once Daily
Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesses*
100 mg
Treatment of Esophageal Candidiasis†
150 mg
Prophylaxis of Candida Infections in HSCT Recipients‡
50 mg
2.2 Dosage for Pediatric Patients 4 Months and Older
The recommended dosage for pediatric patients 4 months of age and older based on indication and weight are shown in Table 2.
Table 2. MYCAMINE Dosage in Pediatric Patients (4 Months of Age and Older) Indication
Dosage for Pediatric Patients
4 Months of Age and Older
30 kg or less
Greater than 30 kg
Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesses
2 mg/kg once daily
(maximum daily dose 100 mg)
Treatment of Esophageal Candidiasis
3 mg/kg once daily
2.5 mg/kg once daily
(maximum daily dose 150 mg)Prophylaxis of Candida Infections in HSCT Recipients
1 mg/kg once daily
(maximum daily dose 50 mg)2.3 Dosage for Pediatric Patients Younger than 4 Months of Age
Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesses without meningoencephalitis and/or ocular dissemination
The recommended dosage is 4 mg/kg once daily.
The safety and effectiveness of MYCAMINE have not been established for the treatment of candidemia with meningoencephalitis and/or ocular dissemination in pediatric patients younger than 4 months of age as a higher dose may be needed [see Use in Specific Populations (8.4), Clinical Pharmacology (12.3) and Microbiology (12.4)].2.4 Directions for Reconstitution, Dilution, and Preparation
Do not mix or co-infuse MYCAMINE with other medications. MYCAMINE has been shown to precipitate when mixed directly with a number of other commonly used medications. Please read this entire section carefully before beginning reconstitution.
Reconstitution
Reconstitute MYCAMINE vials by aseptically adding 5 mL of one of the following compatible solutions:
- •
- 0.9% Sodium Chloride Injection, USP (without a bacteriostatic agent)
- •
- 5% Dextrose Injection, USP
To minimize excessive foaming, gently dissolve the MYCAMINE powder by swirling the vial. Do not vigorously shake the vial. Visually inspect the vial for particulate matter.
MYCAMINE 50 mg vial: after reconstitution each mL contains 10 mg of micafungin.
MYCAMINE 100 mg vial: after reconstitution each mL contains 20 mg of micafungin.Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if there is any evidence of precipitation or foreign matter. Aseptic technique must be strictly observed in all handling since no preservative or bacteriostatic agent is present in MYCAMINE or in the materials specified for reconstitution and dilution.
The reconstituted product should be protected from light and may be stored in the original vial for up to 24 hours at room temperature, 25oC (77oF).
Dilution and Preparation
The diluted solution should be protected from light. It is not necessary to cover the infusion drip chamber or the tubing.
Adult Patients:
- 1.
- Add the appropriate volume of reconstituted MYCAMINE into 100 mL of 0.9% Sodium Chloride Injection, USP or 100 mL of 5% Dextrose Injection, USP.
- 2.
- Appropriately label the bag.
Pediatric Patients
- 1.
- Calculate the total MYCAMINE dose in milligrams (mg) by multiplying the recommended pediatric dose (mg/kg) for a given indication [see Table 2] and the weight of the patient in kilograms (kg).
- 2.
- To calculate the volume (mL) of drug needed, divide the calculated dose (mg) from step 1 by the final concentration of the selected reconstituted vial(s) (either 10 mg/mL for the 50 mg vial or 20 mg/mL for the
100 mg vial), see example below:
Using 50 mg vials:
Divide the calculated mg dose (from step 1) by 10 mg/mL to determine the volume (mL) needed.
OR
Using 100 mg vials:
Divide the calculated mg dose (from step 1) by 20 mg/mL to determine the volume (mL) needed.
- 3.
- Withdraw the calculated volume (mL) of drug needed from the selected concentration and size of reconstituted MYCAMINE vial(s) used in Step 2 (ensure the selected concentration and vial size used to calculate the dose is also used to prepare the infusion).
- 4.
- Add the withdrawn volume of drug (step 3) to a 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP intravenous infusion bag or syringe. Ensure that the final concentration of the solution is between 0.5 mg/mL to 4 mg/mL.
To decrease the risk of infusion reactions, concentrations above 1.5 mg/mL should be administered via central catheter [see Warnings and Precautions (5.5)].
- 5.
- Appropriately label the infusion bag or syringe. For concentrations above 1.5 mg/mL, if required, label to specifically warn to administer the solution via central catheter.
The diluted infusion bag should be protected from light and may be stored for up to 24 hours at room temperature, 25oC (77oF).
MYCAMINE is preservative-free. Discard partially used vials.
2.5 Infusion Volume and Duration
Administer MYCAMINE by intravenous infusion only. Infuse over one hour. More rapid infusions may result in more frequent histamine-mediated reactions [see Warnings and Precautions (5.5)].
Flush an existing intravenous line with 0.9% Sodium Chloride Injection, USP, prior to infusion of MYCAMINE.
Pediatric Patients
MYCAMINE should be infused over one hour. To decrease the risk of infusion reactions, concentrations above
1.5 mg/mL should be administered via central catheter [see Warnings and Precautions (5.5)]. - 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Isolated cases of serious hypersensitivity (anaphylaxis and anaphylactoid) reactions (including shock) have been reported in patients receiving MYCAMINE. If these reactions occur, MYCAMINE infusion should be discontinued and appropriate treatment administered.
5.2 Hematological Effects
Acute intravascular hemolysis and hemoglobinuria was seen in a healthy volunteer during infusion of MYCAMINE (200 mg) and oral prednisolone (20 mg). Cases of significant hemolysis and hemolytic anemia have also been reported in patients treated with MYCAMINE. Patients who develop clinical or laboratory evidence of hemolysis or hemolytic anemia during MYCAMINE therapy should be monitored closely for evidence of worsening of these conditions and evaluated for the risk/benefit of continuing MYCAMINE therapy.
5.3 Hepatic Effects
Laboratory abnormalities in liver function tests have been seen in healthy volunteers and patients treated with MYCAMINE. In some patients with serious underlying conditions who were receiving MYCAMINE along with multiple concomitant medications, clinical hepatic abnormalities have occurred, and isolated cases of significant hepatic impairment, hepatitis, and hepatic failure have been reported. Patients who develop abnormal liver function tests during MYCAMINE therapy should be monitored for evidence of worsening hepatic function and evaluated for the risk/benefit of continuing MYCAMINE therapy.
5.4 Renal Effects
Elevations in BUN and creatinine, and isolated cases of significant renal impairment or acute renal failure have been reported in patients who received MYCAMINE. In fluconazole-controlled trials, the incidence of drug-related renal adverse reactions was 0.4% for MYCAMINE-treated patients and 0.5% for fluconazole-treated patients. Patients who develop abnormal renal function tests during MYCAMINE therapy should be monitored for evidence of worsening renal function.
5.5 Infusion and Injection Site Reactions
Possible histamine-mediated symptoms have been reported with MYCAMINE, including rash, pruritus, facial swelling, and vasodilatation. Slow the infusion rate if infusion reaction occurs [see Dosage and Administration (2.3)].
Injection site reactions, including phlebitis and thrombophlebitis have been reported, at MYCAMINE doses of 50 to 150 mg/day. These reactions tended to occur more often in patients receiving MYCAMINE via peripheral intravenous administration [see Dosage and Administration (2.3) and Adverse Reactions (6.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- •
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- •
- Hematological Effects [see Warnings and Precautions (5.2)]
- •
- Hepatic Effects [see Warnings and Precautions (5.3)]
- •
- Renal Effects [see Warnings and Precautions (5.4)]
- •
- Infusion and Injection Site Reactions [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of MYCAMINE cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
The overall safety of MYCAMINE was assessed in 520 healthy volunteers and 3417 adult and pediatric patients who received single or multiple doses of MYCAMINE across 50 clinical trials, including the invasive candidiasis, esophageal candidiasis and prophylaxis trials. The doses of MYCAMINE administered included doses above and below the recommended doses [see Dosage and Administration (2.1, 2.2)] and ranged from 0.75 mg/kg to 15 mg/kg in pediatric patients and 12.5 mg to 150 mg/day or greater in adults.
Clinical Trials Experience in Adults
In clinical trials with MYCAMINE, 2497/2748 (91%) adult patients experienced at least one adverse reaction.
Candidemia and Other Candida Infections
In a randomized, double-blind trial for the treatment of candidemia and other Candida infections, adverse reactions occurred in 183/200 (92%) and 171/193 (89%) patients in the MYCAMINE 100 mg/day, and caspofungin (70 mg loading dose followed by 50 mg/day dose) treatment groups, respectively. Selected adverse reactions occurring in 5% or more of the patients and more frequently in the MYCAMINE treatment group, are shown in Table 3.
Table 3. Selected* Adverse Reactions in Adult Patients with Candidemia and Other Candida Infections Patient base: all randomized patients who received at least 1 dose of trial drug. Adverse Reactions by
System Organ Class†
MYCAMINE
100 mgn (%)
Caspofungin‡
n (%)
Number of Patients
200
193
Gastrointestinal Disorders
81 (41)
76 (39)
Diarrhea
15 (8)
14 (7)
Vomiting
18 (9)
16 (8)
Metabolism and Nutrition Disorders
77 (39)
73 (38)
Hypoglycemia
12 (6)
9 (5)
Hyperkalemia
10 (5)
5 (3)
General Disorders/Administration Site Conditions
59 (30)
51 (26)
Investigations
36 (18)
37 (19)
Blood Alkaline Phosphatase
Increased11 (6)
8 (4)
Cardiac Disorders
35 (18)
36 (19)
Atrial Fibrillation
5 (3)
0
In a second, supportive, randomized, double-blind trial for the treatment of candidemia and other Candida infections, adverse reactions occurred in 245/264 (93%) and 250/265 (94%) adult and pediatric patients in the MYCAMINE (100 mg/day) and amphotericin B liposome (3 mg/kg/day) treatment groups, respectively. In this trial, the following adverse reactions were reported in patients at least 16 years of age in the MYCAMINE and amphotericin B liposome treatment groups, respectively: nausea (10% vs. 8%), diarrhea (11% vs. 11%), vomiting (13% vs. 9%), abnormal liver tests (4% vs. 3%), increased aspartate aminotransferase (3% vs. 2%), and increased blood alkaline phosphatase
(3% vs. 2%).Esophageal Candidiasis
In a randomized, double-blind study for treatment of esophageal candidiasis, a total of 202/260 (78%) patients who received MYCAMINE 150 mg/day and 186/258 (72%) patients who received intravenous fluconazole 200 mg/day experienced an adverse reaction. Adverse reactions resulting in discontinuation were reported in 17 (7%) MYCAMINE-treated patients; and in 12 (5%) fluconazole-treated patients. Selected treatment-emergent adverse reactions occurring in 5% or more of the patients and more frequently in the MYCAMINE group, are shown in Table 4.
Table 4. Selected* Adverse Reactions in Adult Patients with Esophageal Candidiasis Patient base: all randomized patients who received at least 1 dose of trial drug. Adverse Reactions by
System Organ Class†MYCAMINE
150 mg/day
n (%)
Fluconazole
200 mg/day
n (%)
Number of Patients
260
258
Gastrointestinal Disorders
84 (32)
93 (36)
Diarrhea
27 (10)
29 (11)
Nausea
20 (8)
23 (9)
Vomiting
17 (7)
17 (7)
General Disorders/Administration Site Conditions
52 (20)
45 (17)
Pyrexia
34 (13)
21 (8)
Nervous System Disorders
42 (16)
40 (16)
Headache
22 (9)
20 (8)
Vascular Disorders
54 (21)
21 (8)
Phlebitis
49 (19)
13 (5)
Skin and Subcutaneous Tissue Disorders
36 (14)
26 (10)
Rash
14 (5)
6 (2)
Prophylaxis of Candida Infections in Hematopoietic Stem Cell Transplant Recipients
A double-blind trial was conducted in a total of 882 patients scheduled to undergo an autologous or allogeneic hematopoietic stem cell transplant. The median duration of treatment was 18 days (range 1 to 51 days) in both treatment arms.
All adult patients who received MYCAMINE (382) or fluconazole (409) experienced at least one adverse reaction during the study. Treatment-emergent adverse reactions resulting in MYCAMINE discontinuation were reported in 15 (4%) adult patients; while those resulting in fluconazole discontinuation were reported in 32 (8%). Selected adverse reactions reported in 15% or more of adult patients and more frequently in the MYCAMINE treatment arm, are shown in Table 5.
Table 5. Selected Adverse Reactions in Adult Patients During Prophylaxis of Candida Infection in Hematopoietic Stem Cell Transplant Recipients Patient base: all randomized adult patients who received at least 1 dose of trial drug. System Organ Class
MYCAMINE
50 mg/day
n (%)
Fluconazole
400 mg/day
n (%)
Number of Patients
382
409
Gastrointestinal Disorders
377 (99)
404 (99)
Diarrhea
294 (77)
327 (80)
Nausea
270 (71)
290 (71)
Vomiting
252 (66)
274 (67)
Abdominal Pain
100 (26)
93 (23)
Blood and Lymphatic System Disorders
368 (96)
385 (94)
Neutropenia
288 (75)
297 (73)
Thrombocytopenia
286 (75)
280 (69)
Skin and Subcutaneous Tissue Disorders
257 (67)
275 (67)
Rash
95 (25)
91 (22)
Nervous System Disorders
250 (65)
254 (62)
Headache
169 (44)
154 (38)
Psychiatric Disorders
233 (61)
235 (58)
Insomnia
142 (37)
140 (34)
Anxiety
84 (22)
87 (21)
Cardiac Disorders
133 (35)
138 (34)
Tachycardia
99 (26)
91 (22)
Other selected adverse reactions reported at less than 5% in adult clinical trials are listed below:
- •
- Blood and lymphatic system disorders: coagulopathy, pancytopenia, thrombotic thrombocytopenic purpura
- •
- Cardiac disorders: cardiac arrest, myocardial infarction, pericardial effusion
- •
- General disorders and administration site conditions: infusion reaction, injection site thrombosis
- •
- Hepatobiliary disorders: hepatocellular damage, hepatomegaly, jaundice, hepatic failure
- •
- Immune disorders: hypersensitivity, anaphylactic reaction
- •
- Metabolism and nutrition disorders: hypernatremia, hypokalemia
- •
- Nervous system disorders: convulsions, encephalopathy, intracranial hemorrhage
- •
- Psychiatric disorders: delirium
- •
- Skin and subcutaneous tissue disorders: urticaria
Clinical Trials Experience in Pediatric Patients
The safety of MYCAMINE was assessed in 593 pediatric patients, 425 of whom were 4 months through 16 years of age and 168 of whom were 3 days to less than 4 months of age who received at least one dose of MYCAMINE across 15 clinical trials.
Of the 425 pediatric patients, 4 months through 16 years of age enrolled in 11 clinical trials, 235 (55%) were male, 290 (68%) were white, with the following age distribution: 62 (15%) 4 months to <2 years, 108 (25%) 2 to 5 years, 140 (33%) 6 to 11 years, and 115 (27%) 12 to 16 years of age. The mean treatment duration was 26.1 days. A total of 246 patients received at least one dose of MYCAMINE ranging from 2 to 10 mg/kg. Overall, 388/425 (91%) patients experienced at least one adverse reaction. Adverse reactions occurring in ≥15% or more of micafungin-treated pediatric patients 4 months of age and older are: vomiting (32%), diarrhea (24%), pyrexia (24%), hypokalemia (22%), nausea (21%), mucosal inflammation (19%), thrombocytopenia (19%), abdominal pain (18%), headache (15%), and hypertension (15%).
Two randomized, double-blind active-controlled trials included pediatric patients. In the invasive candidiasis/candidemia trial, the efficacy and safety of MYCAMINE (2 mg/kg/day for patients weighing 40 kg or less and 100 mg/day for patients weighing greater than 40 kg) was compared to amphotericin B liposome (3 mg/kg/day) in 112 pediatric patients. Treatment-emergent adverse reactions occurred in 51/56 (91%) of patients in the MYCAMINE group and 52/56 (93%) of patients in the amphotericin B liposome group. Treatment-emergent adverse reactions resulting in drug discontinuation were reported in 2 (4%) MYCAMINE-treated pediatric patients and in 9 (16%) amphotericin B liposome-treated pediatric patients.
The prophylaxis study in patients undergoing HSCT investigated the efficacy of MYCAMINE (1 mg/kg/day for patients weighing 50 kg or less and 50 mg/day for patients weighing greater than 50 kg) as compared to fluconazole (8 mg/kg/day for patients weighing 50 kg or less and 400 mg/day for patients weighing greater than 50 kg). All 91 pediatric patients experienced at least one treatment-emergent adverse reaction. Three (7%) pediatric patients discontinued MYCAMINE due to adverse reaction, while one (2%) patient discontinued fluconazole.
Selected adverse reactions, occurring in 15% or more of the patients and more frequently in a MYCAMINE group, for the two comparative trials are shown in Table 6.
Table 6. Selected Adverse Reactions in Pediatric Patients with Candidemia and Other Candida Infections (C/IC), and in Hematopoietic Stem-Cell Recipients During Prophylaxis of Candida Infections Adverse Reactions*
C/IC†
Prophylaxis
MYCAMINE
n = 56
n (%)Amphotericin B liposome
n = 56
n (%)MYCAMINE
n = 43
n (%)Fluconazole
n = 48
n (%)Gastrointestinal disorders
22 (40)
18 (32)
43 (100)
45 (94)
Vomiting
10 (18)
8 (14)
28 (65)
32 (67)
Diarrhea
4 (7)
5 (9)
22 (51)
31 (65)
Nausea
4 (7)
4 (7)
30 (70)
25 (52)
Abdominal pain
2 (4)
2 (4)
15 (35)
12 (25)
Abdominal distension
1 (2)
1 (2)
8 (19)
6 (13)
General disorders and administration site conditions
14 (25)
14 (25)
41 (95)
46 (96)
Pyrexia
5 (9)
9 (16)
26 (61)
31 (65)
Infusion-related reaction
0
3 (5)
7 (16)
4 (8)
Skin and subcutaneous tissue disorders
11 (20)
8 (14)
33 (77)
38 (79)
Pruritus
0
1 (2)
14 (33)
15 (31)
Rash
1 (2)
1 (2)
13 (30)
13 (27)
Urticaria
0
1 (2)
8 (19)
4 (8)
Respiratory, thoracic and mediastinal disorders
9 (16)
13 (23)
30 (70)
33 (69)
Epistaxis
0
0
4 (9)
8 (17)
Blood and lymphatic system disorders
17 (30)
13 (23)
40 (93)
44 (92)
Thrombocytopenia
5 (9)
3 (5)
31 (72)
37 (77)
Neutropenia
3 (5)
4 (7)
33 (77)
34 (71)
Anemia
10 (18)
6 (11)
22 (51)
24 (50)
Febrile neutropenia
0
0
7 (16)
7 (15)
Investigations
12 (21)
8 (14)
24 (56)
25 (52)
Alanine aminotransferase
increased0
0
7 (16)
1 (2)
Urine output decreased
0
0
10 (23)
8 (17)
Cardiac disorders
7 (13)
3 (5)
10 (23)
17 (35)
Tachycardia
2 (4)
1 (2)
7 (16)
12 (25)
Renal and urinary disorders
4 (7)
4 (7)
16 (37)
15 (31)
Hematuria
0
0
10 (23)
7 (15)
Psychiatric disorders
3 (5)
1 (2)
20 (47)
9 (19)
Anxiety
0
0
10 (23)
3 (6)
Other clinically significant adverse reactions reported at less than 15% in pediatric clinical trials are listed below:
- •
- Hepatobiliary disorders: hyperbilirubinemia
- •
- Investigations: liver tests abnormal
- •
- Renal Disorders: renal failure
Clinical Trials Experience in Pediatric Patients Younger than 4 Months of Age
The safety of MYCAMINE was assessed in 168 pediatric patients younger than 4 months of age who received varying doses of MYCAMINE in 9 clinical trials. The mean treatment duration was 16.6 days. A total of 59 patients received MYCAMINE at doses ≤4 mg/kg/day and 109 patients received MYCAMINE doses >4 mg/kg/day [5 to 15 mg/kg/day (approximately 1.3 to 3.8 times the recommended dosage in pediatric patients less than 4 months old)].
The adverse reaction profile of MYCAMINE in pediatric patients younger than 4 months of age was generally comparable to that of pediatric patients 4 months of age and older and adults. The most frequent adverse reactions (≥15%) in pediatric patients younger than 4 months old receiving a MYCAMINE dose of approximately 4 mg/kg/day included hypokalemia (25%), thrombocytopenia (25%), acidosis (20%), sepsis (20%), anemia (15%), oxygen saturation decreased (15%), and vomiting (15%). No new safety signals were seen in patients who received 5 to 15 mg/kg/day [see Use in Specific Populations (8.4)].
Additional clinically significant adverse reactions reported in less than 15% of pediatric patients younger than 4 months of age who received approximately 4 mg/kg/day are listed below:
- •
- Blood and Lymphatic System Disorders: leukocytosis, thrombocytosis, coagulation disorder neonatal
- •
- Gastrointestinal Disorders: hematochezia, intestinal perforation, ascites, ileus, intestinal infarction, diarrhea, abdominal distension
- •
- General Disorders and Administration Site Conditions: peripheral swelling, generalized edema, pyrexia, infusion site extravasation, edema neonatal
- •
- Hepatobiliary Disorders: hyperbilirubinemia
- •
- Investigations: blood lactate dehydrogenase increased, blood urea increased, ECG QRS complex prolonged
- •
- Vascular Disorders: neonatal hypotension, thrombophlebitis
- •
- Musculoskeletal and connective tissue disorders: hypertonia neonatal
- •
- Respiratory, thoracic and mediastinal disorders: pleural effusion, respiratory failure, neonatal aspiration, respiratory distress
- •
- Metabolism and nutrition disorders: hyperglycemia, dehydration, hypocalcemia, hypermagnesemia
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of micafungin for injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- •
- Blood and lymphatic system disorders: disseminated intravascular coagulation
- •
- Hepatobiliary disorders: hepatic disorder
- •
- Renal and urinary disorders: renal impairment
- •
- Skin and subcutaneous tissue disorders: Stevens-Johnson syndrome, toxic epidermal necrolysis
- •
- Vascular disorders: shock
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on MYCAMINE
CYP3A4, CYP2C9 and CYP2C19 Inhibitors
Co-administration of MYCAMINE with cyclosporine, itraconazole, voriconazole and fluconazole did not alter the pharmacokinetics of MYCAMINE.
CYP2C19 and CYP3A4 Inducer
Co-administration of MYCAMINE with rifampin and ritonavir did not alter the pharmacokinetics of MYCAMINE.
Co-administration of MYCAMINE with Other Drugs
Co-administration of MYCAMINE with mycophenolate mofetil (MMF), amphotericin B, tacrolimus, prednisolone, sirolimus and nifedipine did not alter the pharmacokinetics of MYCAMINE.
7.2 Effect of MYCAMINE on Other Drugs
CYP3A4 Substrates
There was no effect of single or multiple doses of MYCAMINE on cyclosporine, tacrolimus, prednisolone, voriconazole and fluconazole pharmacokinetics.
Sirolimus AUC was increased by 21% with no effect on Cmax in the presence of steady-state MYCAMINE compared with sirolimus alone. Nifedipine AUC and Cmax were increased by 18% and 42%, respectively, in the presence of steady-state MYCAMINE compared with nifedipine alone. Itraconazole AUC and Cmax were increased by 22% and 11%, respectively. Patients receiving sirolimus, nifedipine, and itraconazole in combination with MYCAMINE should be monitored for sirolimus, nifedipine, and itraconazole toxicity and the sirolimus, nifedipine, and itraconazole dosage should be reduced if necessary.
UDP-Glycosyltransferase Substrate
Co-administration of mycophenolate mofetil (MMF) with MYCAMINE did not alter the pharmacokinetics of MMF.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies, MYCAMINE may cause fetal harm when administered to a pregnant woman (see Data). There is insufficient human data on the use of MYCAMINE in pregnant women to inform a drug-associated risk of adverse developmental outcomes. In animal reproduction studies, intravenous administration of micafungin sodium to pregnant rabbits during organogenesis at doses four times the maximum recommended human dose resulted in visceral abnormalities and increased abortion (see Data). Advise pregnant women of the risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Animal Data
In an embryo-fetal toxicity study in pregnant rabbits, intravenous administration of micafungin sodium during organogenesis (days 6 to 18 of gestation) resulted in fetal visceral abnormalities and abortion at 32 mg/kg, a dose equivalent to four times the recommended human dose based on body surface area comparisons. Visceral abnormalities included abnormal lobation of the lung, levocardia, retrocaval ureter, anomalous right subclavian artery, and dilatation of the ureter.
8.2 Lactation
Risk Summary
There are no data on the presence of micafungin in human milk, the effects on the breast-fed infant or the effects on milk production. Micafungin was present in the milk of lactating rats following intravenous administration. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for MYCAMINE, and any potential adverse effects on the breast-fed child from MYCAMINE, or from the underlying maternal condition.
8.4 Pediatric Use
Pediatric Patients 4 Months of Age and Older
The safety and effectiveness of MYCAMINE for the treatment of esophageal candidiasis, candidemia, acute disseminated candidiasis, Candida peritonitis and abscesses, esophageal candidiasis, and for prophylaxis of Candida infections in patients undergoing HSCT have been established in pediatric patients 4 months of age and older. Use of MYCAMINE for these indications and in this age group is supported by evidence from adequate and well-controlled studies in adult and pediatric patients with additional pharmacokinetic and safety data in pediatric patients 4 months of age and older [see Indications and Usage (1), Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)].
Pediatric Patients Younger than 4 Months of Age
Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesses Without Meningoencephalitis and/or Ocular Dissemination in Pediatric Patients Younger Than 4 Months of Age
The safety and effectiveness of MYCAMINE for the treatment of candidemia, acute disseminated candidiasis, Candida peritonitis and abscesses without meningoencephalitis and/or ocular dissemination at a dosage of 4 mg/kg once daily have been established in pediatric patients younger than 4 months of age. This use and dosage of MYCAMINE are supported by evidence from adequate and well-controlled studies in adult and pediatric patients 4 months of age and older with additional pharmacokinetic and safety data in pediatric patients younger than 4 months of age [see Adverse Reactions (6.1) and Clinical Pharmacology (12.3)].
Treatment of Candidemia, Acute Disseminated Candidiasis, Candida Peritonitis and Abscesses With Meningoencephalitis and/or Ocular Dissemination in Pediatric Patients Younger Than 4 Months of Age
The safety and effectiveness of MYCAMINE have not been established for the treatment of candidemia with meningoencephalitis and/or ocular dissemination in pediatric patients younger than 4 months of age.
In a rabbit model of hematogenous Candida meningoencephalitis (HCME) with Candida albicans (minimum inhibitory concentration of 0.125 mcg/mL), a decrease in mean fungal burden in central nervous system (CNS) compartments assessed as the average of combined fungal burden in the cerebrum, cerebellum, and spinal cord relative to untreated controls, was observed with increasing micafungin dosages administered once daily for 7 days. Data from the rabbit model suggest that a micafungin dose regimen of 4 mg/kg once daily is inadequate to treat meningoencephalitis and that a dose regimen of approximately 10 to 25 mg/kg once daily may be necessary to lower fungal burden in the CNS in pediatric patients younger than 4 months of age [see Microbiology (12.4)]. In this rabbit model, micafungin concentrations could not be reliably detected in cerebrospinal fluid (CSF). Due to limitations of the study design, the clinical significance of a decreased CNS fungal burden in the rabbit HCME model is uncertain.
A randomized controlled trial evaluated a MYCAMINE dose regimen of 10 mg/kg once daily in pediatric patients younger than 4 months of age with suspected or proven Candida meningoencephalitis. Fungal-free survival at 1 week after end of therapy was observed in 60% of MYCAMINE-treated vs. 70% of amphotericin B-treated patients, and all-cause mortality was 15% vs. 10%, respectively. However, because this study was terminated early and enrolled only 30 pediatric patients younger than 4 months of age (20 treated with MYCAMINE and 10 treated with amphotericin B) which was 13% of the planned enrollment for the study, no conclusions can be drawn regarding efficacy of MYCAMINE at this dose regimen.
In six uncontrolled, open-label studies, and a neonatal intensive care unit (ICU) medical records database, pediatric patients younger than 4 months of age with suspected Candida meningoencephalitis or disseminated candidemia received MYCAMINE at dose regimens ranging from 5 to 15 mg/kg once daily. Across the entire MYCAMINE development program, only 6 pediatric patients with proven Candida meningoencephalitis were treated with dosages of 2 mg/kg, 8 mg/kg and 10 mg/kg once daily. Micafungin was detected in the CSF of pediatric patients with suspected Candida meningoencephalitis. No conclusions regarding the efficacy of a particular dosage of MYCAMINE or the penetration of micafungin into the CSF can be drawn due to limitations of the data, including but not limited to, multiple confounding factors, variable study designs, and limited numbers of patients. No new safety signals were observed with the use of MYCAMINE at dosages of 5 to 15 mg/kg once daily in pediatric patients younger than 4 months of age, and there was no discernible dose-response for adverse events.
Although the dosage for the treatment of candidemia with meningoencephalitis has not been established, antifungal activity in various CNS compartments in the rabbit HCME model and limited clinical trial data suggest that in patients younger than 4 months of age, dose regimens 10 mg/kg once daily or higher may be necessary for the treatment of candidemia with meningoencephalitis. Safety data from clinical studies for MYCAMINE at dose regimens of 10 to 15 mg/kg once daily in pediatric patients younger than 4 months of age did not reveal new safety signals.
Treatment of Esophageal Candidiasis and Prophylaxis of Candida Infections in Patients Undergoing Hematopoietic Stem Cell Transplantation in Pediatric Patients Younger Than 4 Months of Age
The safety and effectiveness of MYCAMINE in pediatric patients younger than 4 months of age have not been established for the:
- •
- Treatment of esophageal candidiasis
- •
- Prophylaxis of Candida infections in patients undergoing hematopoietic stem cell transplantation
8.5 Geriatric Use
A total of 418 subjects in clinical studies of MYCAMINE were 65 years of age and older, and 124 subjects were 75 years of age and older. No overall differences in safety and effectiveness were observed between these subjects and younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
The exposure and disposition of a 50 mg MYCAMINE dose administered as a single 1-hour infusion to 10 healthy subjects aged 66 to 78 years were not significantly different from those in 10 healthy subjects aged 20 to 24 years. No dose adjustment is necessary for the elderly.
8.6 Use in Patients with Renal Impairment
MYCAMINE does not require dose adjustment in patients with renal impairment. Supplementary dosing should not be required following hemodialysis [see Clinical Pharmacology (12.3)].
8.7 Use in Patients with Hepatic Impairment
Dose adjustment of MYCAMINE is not required in patients with mild, moderate, or severe hepatic impairment [see Clinical Pharmacology (12.3)].
8.8 Race and Gender
No dose adjustment of MYCAMINE is required based on gender or race. After 14 daily doses of 150 mg to healthy subjects, micafungin AUC in women was greater by approximately 23% compared with men, due to smaller body weight. No notable differences among white, black, and Hispanic subjects were seen. The micafungin AUC was greater by 19% in Japanese subjects compared to blacks, due to smaller body weight.
- 9 DRUG ABUSE AND DEPENDENCE
-
10 OVERDOSAGE
MYCAMINE is highly protein bound and, therefore, is not dialyzable. No cases of MYCAMINE overdosage have been reported. Repeated daily doses up to 8 mg/kg (maximum total dose of 896 mg) in adult patients, up to 6 mg/kg in pediatric patients 4 months of age and older, and up to 15 mg/kg in pediatric patients younger than 4 months of age have been administered in clinical trials with no reported dose-limiting toxicity [see Adverse Reactions (6.1) and Use in Specific Populations (8.4)].
-
11 DESCRIPTION
MYCAMINE is a sterile, lyophilized product for intravenous (IV) infusion that contains micafungin sodium. Micafungin sodium is a semisynthetic lipopeptide (echinocandin) synthesized by a chemical modification of a fermentation product of Coleophoma empetri F-11899. Micafungin inhibits the synthesis of 1,3-beta-D-glucan, an integral component of the fungal cell wall.
Each single-dose vial contains 50 mg micafungin (equivalent to 50.86 mg micafungin sodium) or 100 mg micafungin (equivalent to 101.73 mg micafungin sodium), 200 mg lactose monohydrate, with citric acid and/or sodium hydroxide (used for pH adjustment). MYCAMINE must be diluted with 0.9% Sodium Chloride Injection, USP, or 5% Dextrose Injection, USP [see Dosage and Administration (2)]. Following reconstitution with 0.9% Sodium Chloride Injection, USP, the resulting pH of the solution is between 5-7.
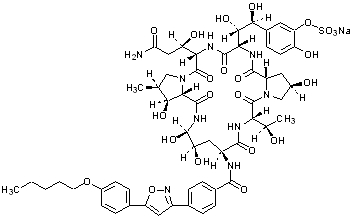
Micafungin sodium is chemically designated as:
Pneumocandin A0,1-[(4R,5R)-4,5-dihydroxy-N2-[4-[5-[4-(pentyloxy) phenyl]-3-isoxazolyl]benzoyl]-L-ornithine]-4-[(4S)-4-hydroxy-4-[4-hydroxy-3-(sulfooxy)phenyl]-L-threonine]-, monosodium salt.
The chemical structure of micafungin sodium is:
The empirical/molecular formula is C56H70N9NaO23S and the formula weight is 1292.26.
Micafungin sodium is a light-sensitive, hygroscopic white powder that is freely soluble in water, isotonic sodium chloride solution, N,N-dimethylformamide and dimethylsulfoxide, slightly soluble in methyl alcohol, and practically insoluble in acetonitrile, ethyl alcohol (95%), acetone, diethyl ether and n-hexane.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Micafungin is a member of the echinocandin class of antifungal agents [see Microbiology (12.4)].
12.2 Pharmacodynamics
The pharmacodynamics of micafungin related to hematogenous Candida meningoencephalitis are described in other sections of the prescribing information [see Use in Specific Populations (8.4) and Microbiology (12.4)].
12.3 Pharmacokinetics
Adults
The pharmacokinetics of micafungin were determined in healthy subjects, hematopoietic stem cell transplant recipients, and patients with esophageal candidiasis up to a maximum daily dose of 8 mg/kg body weight.
The relationship of area under the concentration-time curve (AUC) to micafungin dose was linear over the daily dose range of 50 mg to 150 mg and 3 mg/kg to 8 mg/kg body weight. Typically, 85% of the steady-state concentration is achieved after three daily MYCAMINE doses.
Steady-state pharmacokinetic parameters in relevant patient populations after repeated daily administration are presented in Table 7.
Table 7. Pharmacokinetic Parameters of Micafungin in Adult Patients Population
n
Dose (mg)
Pharmacokinetic Parameters
(Mean ± Standard Deviation)
Cmax
(mcg/mL)
AUC0-24*
(mcg·h/mL)
t½
(h)
Cl
(mL/min/kg)
Patients with IC†
[Day 1]
20
100
5.7 ± 2.2
83 ± 51
14.5 ± 7.0
0.359 ± 0.179
[Steady-State]
20
100
10.1 ± 4.4
97 ± 29
13.4 ± 2.0
0.298 ± 0.115
HIV‡- Positive
Patients with EC§
[Day 1]
20
20
14
50
100
150
4.1 ± 1.4
8.0 ± 2.4
11.6 ± 3.1
36 ± 9
108 ± 31
151 ± 45
14.9 ± 4.3
13.8 ± 3.0
14.1 ± 2.6
0.321 ± 0.098
0.327 ± 0.093
0.340 ± 0.092
[Day 14 or 21]
20
20
14
50
100
150
5.1 ± 1.0
10.1 ± 2.6
16.4 ± 6.5
54 ± 13
115 ± 25
167 ± 40
15.6 ± 2.8
16.9 ± 4.4
15.2 ± 2.2
0.300 ± 0.063
0.301 ± 0.086
0.297 ± 0.081
HSCT¶ Recipients
[Day 7]
8
10
8
8
per kg
3
4
6
8
21.1 ± 2.84
29.2 ± 6.2
38.4 ± 6.9
60.8 ± 26.9
234 ± 34
339 ± 72
479 ± 157
663 ± 212
14.0 ± 1.4
14.2 ± 3.2
14.9 ± 2.6
17.2 ± 2.3
0.214 ± 0.031
0.204 ± 0.036
0.224 ± 0.064
0.223 ± 0.081
Pediatric Patients 4 Months of Age and Older
Micafungin pharmacokinetics in 229 pediatric patients 4 months through 16 years of age were characterized using population pharmacokinetics. Micafungin exposure was dose proportional across the dose and age range studied.
Table 8. Summary (Mean +/- Standard Deviation) of Micafungin Pharmacokinetics in Pediatric Patients 4 Months of Age and Older (Steady-State) Body weight group
N
Dose*
mg/kgCmax.ss†
(mcg/mL)AUC.ss†
(mcg·h/mL)t½‡
(h)CL‡
(mL/min/kg)30 kg or less
149
1.0
7.1 +/- 4.7
55 +/- 16
12.5 +/- 4.6
0.328 +/- 0.091
2.0
14.2 +/- 9.3
109 +/- 31
3.0
21.3 +/- 14.0
164 +/- 47
Greater than 30 kg
80
1.0
8.7 +/- 5.6
67 +/- 17
13.6 +/- 8.8
0.241 +/- 0.061
2.0
17.5 +/- 11.2
134 +/- 33
2.5
23.0 +/- 14.5
176 +/- 42
Pediatric Patients Younger than 4 Months of Age
Micafungin pharmacokinetic data in 103 pediatric patients less than 4 months of age were assessed using population pharmacokinetics. Predicted micafungin AUC estimates were dose proportional across the dose regimens and age ranges studied. The body weight-normalized micafungin clearance in pediatric patients less than 4 months of age is higher than the body weight-normalized micafungin clearance in older pediatric patients greater than 4 months of age and adults. Administration of 4 mg/kg once daily micafungin to pediatric patients less than 4 months of age produces a mean (SD) steady-state AUC of 131 (50) mcg·h/mL, which is comparable to the steady-state AUC in pediatric patients 4 months of age and older administered micafungin 2 mg/kg/day and adults administered 100 mg once daily.
Specific Populations
Adult Patients with Renal Impairment
MYCAMINE does not require dose adjustment in patients with renal impairment. A single 1-hour infusion of 100 mg MYCAMINE was administered to 9 adult subjects with severe renal impairment (creatinine clearance less than 30 mL/min) and to 9 age-, gender-, and weight-matched subjects with normal renal function (creatinine clearance greater than 80 mL/min). The maximum concentration (Cmax) and AUC were not significantly altered by severe renal impairment.
Since micafungin is highly protein bound, it is not dialyzable. Supplementary dosing should not be required following hemodialysis.
Adult Patients with Hepatic Impairment
- •
- A single 1-hour infusion of 100 mg MYCAMINE was administered to 8 adult subjects with moderate hepatic impairment (Child-Pugh score 7 to 9) and 8 age-, gender-, and weight-matched subjects with normal hepatic function. The Cmax and AUC values of micafungin were lower by approximately 22% in subjects with moderate hepatic impairment compared to normal subjects. This difference in micafungin exposure does not require dose adjustment of MYCAMINE in patients with moderate hepatic impairment.
- •
- A single 1-hour infusion of 100 mg MYCAMINE was administered to 8 adult subjects with severe hepatic impairment (Child-Pugh score 10 to 12) and 8 age-, gender-, ethnic- and weight-matched subjects with normal hepatic function. The mean Cmax and AUC values of micafungin were lower by approximately 30% in subjects with severe hepatic impairment compared to normal subjects. The mean Cmax and AUC values of M-5 metabolite were approximately 2.3-fold higher in subjects with severe hepatic impairment compared to normal subjects; however, this exposure (parent and metabolite) was comparable to that in patients with systemic Candida infection. Therefore, no MYCAMINE dose adjustment is necessary in patients with severe hepatic impairment.
Distribution
The mean ± standard deviation volume of distribution of micafungin at terminal phase was 0.39 ± 0.11 L/kg body weight when determined in adult patients with esophageal candidiasis at the dose range of 50 mg to 150 mg.
Micafungin is highly (greater than 99%) protein bound in vitro, independent of plasma concentrations over the range of 10 to 100 mcg/mL. The primary binding protein is albumin; however, micafungin, at therapeutically relevant concentrations, does not competitively displace bilirubin binding to albumin. Micafungin also binds to a lesser extent to α1-acid-glycoprotein.
Micafungin is neither a substrate nor an inhibitor of P-glycoprotein.
Metabolism
Micafungin is metabolized to M-1 (catechol form) by arylsulfatase, with further metabolism to M-2 (methoxy form) by catechol-O-methyltransferase. M-5 is formed by hydroxylation at the side chain (ω-1 position) of micafungin catalyzed by cytochrome P450 (CYP) isozymes. Even though micafungin is a substrate for and a weak inhibitor of CYP3A in vitro, hydroxylation by CYP3A is not a major pathway for micafungin metabolism in vivo. Micafungin is neither a P-glycoprotein substrate nor inhibitor in vitro.
In four healthy volunteer studies, the ratio of metabolite to parent exposure (AUC) at a dose of 150 mg/day was 6% for M-1, 1% for M-2, and 6% for M-5. In patients with esophageal candidiasis, the ratio of metabolite to parent exposure (AUC) at a dose of 150 mg/day was 11% for M-1, 2% for M-2, and 12% for M-5.
Excretion
The excretion of radioactivity following a single intravenous dose of 14C-micafungin sodium for injection (25 mg) was evaluated in healthy volunteers. At 28 days after administration, mean urinary and fecal recovery of total radioactivity accounted for 82.5% (76.4% to 87.9%) of the administered dose. Fecal excretion is the major route of elimination (total radioactivity at 28 days was 71% of the administered dose).
12.4 Microbiology
Mechanism of Action
Micafungin inhibits the synthesis of 1,3-beta-D-glucan, an essential component of fungal cell walls, which is not present in mammalian cells.
Activity in Animal Models of Candidiasis
Activity of micafungin has been demonstrated in both mucosal and disseminated murine and rabbit models of candidiasis. Micafungin administered to immunocompetent or immunosuppressed mice or rabbits with disseminated candidiasis prolonged survival (mice) and/or decreased the fungal burden in different organs including brain in a dose-dependent manner (mice and rabbits). Overall, antifungal activity of micafungin was demonstrated in the brain and eye tissues of nonneutropenic rabbits with HCME infected with a micafungin-sensitive strain of C. albicans; however, the activity varied in different central nervous system and ocular compartments. In the cerebrum, culture negativity was achieved at a micafungin dose regimen of 32 mg/kg once daily for 7 days; whereas, in spinal cord, vitreous humor, and choroid, culture negativity was achieved at micafungin dose regimens of 24 to 32 mg/kg once daily. Compared to untreated animals, micafungin dose regimens between 8 and 24 mg/kg once daily reduced fungal burden in the cerebrum and cerebellum. When cerebrum, cerebellum and spinal cord data were combined, a decrease in fungal burden relative to untreated controls was evident at micafungin dose regimens between 16 and 32 mg/kg once daily [see Use in Specific Populations (8.4)].
Resistance
There have been reports of clinical failures in patients receiving MYCAMINE therapy due to the development of drug resistance. Some of these reports have identified specific mutations in the FKS protein component of the glucan synthase enzyme that are associated with higher MICs and breakthrough infection.
Antimicrobial Activity
Micafungin has been shown to be active against most isolates of the following Candida species, both in vitro and in clinical infections [see Indications and Usage (1)]:
Candida albicans
Candida glabrata
Candida guilliermondii
Candida krusei
Candida parapsilosis
Candida tropicalisSusceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Hepatic carcinomas and adenomas were observed in a 6-month intravenous toxicology study with an 18-month recovery period of micafungin sodium in rats designed to assess the reversibility of hepatocellular lesions.
Rats administered micafungin sodium for 3 months at 32 mg/kg/day (corresponding to 8 times the highest recommended human dose [150 mg/day], based on AUC comparisons), exhibited colored patches/zones, multinucleated hepatocytes and altered hepatocellular foci after 1 or 3-month recovery periods, and adenomas were observed after a 21-month recovery period. Rats administered micafungin sodium at the same dose for 6 months exhibited adenomas after a 12-month recovery period; after an 18-month recovery period, an increased incidence of adenomas was observed and, additionally, carcinomas were detected. A lower dose of micafungin sodium (equivalent to 5 times the human AUC) in the 6-month rat study resulted in a lower incidence of adenomas and carcinomas following 18 months recovery. The duration of micafungin dosing in these rat studies (3 or 6 months) exceeds the usual duration of MYCAMINE dosing in patients, which is typically less than 1 month for treatment of esophageal candidiasis, but dosing may exceed 1 month for Candida prophylaxis.
Although the increase in carcinomas in the 6-month rat study did not reach statistical significance, the persistence of altered hepatocellular foci subsequent to MYCAMINE dosing, and the presence of adenomas and carcinomas in the recovery periods suggest a causal relationship between micafungin sodium, altered hepatocellular foci, and hepatic neoplasms. Whole-life carcinogenicity studies of MYCAMINE in animals have not been conducted, and it is not known whether the hepatic neoplasms observed in treated rats also occur in other species, or if there is a dose threshold for this effect.
Micafungin sodium was not mutagenic or clastogenic when evaluated in a standard battery of in vitro and in vivo tests (i.e., bacterial reversion - S. typhimurium, E. coli; chromosomal aberration; intravenous mouse micronucleus).
Male rats treated intravenously with micafungin sodium for 9 weeks showed vacuolation of the epididymal ductal epithelial cells at or above 10 mg/kg (about 0.6 times the recommended clinical dose for esophageal candidiasis, based on body surface area comparisons). Higher doses (about twice the recommended clinical dose, based on body surface area comparisons) resulted in higher epididymis weights and reduced numbers of sperm cells. In a 39-week intravenous study in dogs, seminiferous tubular atrophy and decreased sperm in the epididymis were observed at 10 and 32 mg/kg, doses equal to about 2 and 7 times the recommended clinical dose, based on body surface area comparisons. There was no impairment of fertility in animal studies with micafungin sodium.
13.2 Animal Toxicology and/or Pharmacology
High doses of micafungin sodium (5 to 8 times the highest recommended human dose, based on AUC comparisons) have been associated with irreversible changes to the liver when administered for 3 or 6 months, and these changes may be indicative of pre-malignant processes [see Nonclinical Toxicology (13.1)].
-
14 CLINICAL STUDIES
14.1 Treatment of Candidemia and Other Candida Infections in Adult and Pediatric Patients 4 Months of Age and Older
Two dose levels of MYCAMINE were evaluated in a randomized, double-blind study to determine the efficacy and safety versus caspofungin in patients with invasive candidiasis and candidemia. Patients were randomized to receive once daily intravenous infusions (IV) of MYCAMINE, either 100 mg/day or 150 mg/day or caspofungin (70 mg loading dose followed by 50 mg maintenance dose). Patients in both study arms were permitted to switch to oral fluconazole after at least 10 days of intravenous therapy, provided they were non-neutropenic, had improvement or resolution of clinical signs and symptoms, had a Candida isolate which was susceptible to fluconazole, and had documentation of 2 negative cultures drawn at least 24 hours apart. Patients were stratified by APACHE II score (20 or less or greater than 20) and by geographic region. Patients with Candida endocarditis were excluded from this analysis. Outcome was assessed by overall treatment success based on clinical (complete resolution or improvement in attributable signs and symptoms and radiographic abnormalities of the Candida infection and no additional antifungal therapy) and mycological (eradication or presumed eradication) response at the end of IV therapy. Deaths that occurred during IV study drug therapy were treated as failures.
In this study, 111/578 (19.2%) of the patients had baseline APACHE II scores of greater than 20, and 50/578 (8.7%) were neutropenic at baseline (absolute neutrophil count less than 500 cells/mm3). Outcome, relapse and mortality data are shown for the recommended dose of MYCAMINE (100 mg/day) and caspofungin in Table 9.
Table 9. Efficacy Analysis: Treatment Success in Patients in Study 03-0-192 with Candidemia and Other Candida Infections - *
- 70 mg loading dose on day 1 followed by 50 mg/day thereafter (caspofungin).
- †
- All patients who received at least one dose of study medication and had documented invasive candidiasis or candidemia. Patients with Candida endocarditis were excluded from the analyses.
- ‡
- A patient may have had greater than 1 organ of dissemination.
- §
- A patient may have had greater than 1 baseline infection species.
- ¶
- All patients who had a culture-confirmed relapse or required systemic antifungal therapy in the post-treatment period for a suspected or proven Candida infection. Also includes patients who died or were not assessed in follow-up.
MYCAMINE 100 mg/day
n (%)
% treatment difference
(95% CI)
Caspofungin 70/50 mg/day*
n (%)
Treatment Success at End of IV Therapy†
135/191 (70.7)
7.4
(-2.0, 16.3)
119/188 (63.3)
Success in Patients with Neutropenia at Baseline
14/22 (63.6)
5/11 (45.5)
Success by Site of Infection
Candidemia
116/163 (71.2)
103/161 (64)
Abscess
4/5 (80)
5/9 (55.6)
Acute Disseminated‡
6/13 (46.2)
5/9 (55.6)
Endophthalmitis
1/3
1/1
Chorioretinitis
0/3
0
Skin
1/1
0
Kidney
2/2
1/1
Pancreas
1/1
0
Peritoneum
1/1
0
Lung/Skin
0/1
0
Lung/Spleen
0/1
0
Liver
0
0/2
Intraabdominal abscess
0
3/5
Chronic Disseminated
0/1
0
Peritonitis
4/6 (66.7)
2/5 (40)
Success by Organism§
C. albicans
57/81 (70.4)
45/73 (61.6)
C. glabrata
16/23 (69.6)
19/31 (61.3)
C. tropicalis
17/27 (63)
22/29 (75.9)
C. parapsilosis
21/28 (75)
22/39 (56.4)
C. krusei
5/8 (62.5)
2/3 (66.7)
C. guilliermondii
1/2
0/1
C. lusitaniae
2/3 (66.7)
2/2
Relapse through 6 Weeks¶
Overall
49/135 (36.3)
44/119 (37)
Culture-confirmed relapse
5
4
Required systemic antifungal therapy
11
5
Died during follow-up
17
16
Not assessed
16
19
Overall study mortality
58/200 (29)
51/193 (26.4)
Mortality during IV therapy
28/200 (14)
27/193 (14)
In two cases of ophthalmic involvement assessed as failures in the above table due to missing evaluation at the end of IV treatment with MYCAMINE, therapeutic success was documented during protocol-defined oral fluconazole therapy.
14.2 Treatment of Esophageal Candidiasis in Adult and Pediatric Patients 4 Months of Age and Older
In two controlled trials involving 763 patients with esophageal candidiasis, 445 adults with endoscopically-proven candidiasis received MYCAMINE, and 318 received fluconazole for a median duration of 14 days (range 1 to 33 days).
MYCAMINE was evaluated in a randomized, double-blind study which compared MYCAMINE 150 mg/day (n = 260) to intravenous fluconazole 200 mg/day (n = 258) in adults with endoscopically-proven esophageal candidiasis. Most patients in this study had HIV infection, with CD4 cell counts less than 100 cells/mm3. Outcome was assessed by endoscopy and by clinical response at the end of treatment. Endoscopic cure was defined as endoscopic grade 0, based on a scale of 0 to 3. Clinical cure was defined as complete resolution in clinical symptoms of esophageal candidiasis (dysphagia, odynophagia, and retrosternal pain). Overall therapeutic cure was defined as both clinical and endoscopic cure. Mycological eradication was determined by culture, and by histological or cytological evaluation of esophageal biopsy or brushings obtained endoscopically at the end of treatment. As shown in Table 10, endoscopic cure, clinical cure, overall therapeutic cure, and mycological eradication were comparable for patients in the MYCAMINE and fluconazole treatment groups.
Table 10. Endoscopic, Clinical, and Mycological Outcomes for Esophageal Candidiasis at End-of-Treatment - *
- Endoscopic and clinical outcome were measured in the modified intent-to-treat population, including all randomized patients who received 1 or more doses of study treatment. The mycological outcome was determined in the per protocol (evaluable) population, including patients with confirmed esophageal candidiasis who received at least 10 doses of study drug, and had no major protocol violations.
- †
- Calculated as MYCAMINE – fluconazole.
Treatment Outcome*
MYCAMINE
150 mg/day
n = 260
Fluconazole
200 mg/day
n = 258
% Difference†
(95% CI)
Endoscopic Cure
228 (87.7%)
227 (88.0%)
-0.3% (-5.9, +5.3)
Clinical Cure
239 (91.9%)
237 (91.9%)
0.06% (-4.6, +4.8)
Overall Therapeutic Cure
223 (85.8%)
220 (85.3%)
0.5% (-5.6, +6.6)
Mycological Eradication
141/189 (74.6%)
149/192 (77.6%)
-3.0% (-11.6, +5.6)
Most patients (96%) in this study had C. albicans isolated at baseline. The efficacy of MYCAMINE was evaluated in less than 10 patients with Candida species other than C. albicans, most of which were isolated concurrently with C. albicans.
Relapse was assessed at 2 and 4 weeks post-treatment in patients with overall therapeutic cure at end of treatment. Relapse was defined as a recurrence of clinical symptoms or endoscopic lesions (endoscopic grade greater than 0). There was no statistically significant difference in relapse rates at either 2 weeks or through 4 weeks post-treatment for patients in the MYCAMINE and fluconazole treatment groups, as shown in Table 11.
Table 11. Relapse of Esophageal Candidiasis at Week 2 and through Week 4 Post-Treatment in Patients with Overall Therapeutic Cure at the End of Treatment Relapse
MYCAMINE
150 mg/day
n = 223
Fluconazole
200 mg/day
n = 220
% Difference*
(95% CI)
Relapse† at Week 2
40 (17.9%)
30 (13.6%)
4.3% (-2.5, 11.1)
Relapse† through Week 4 (cumulative)
73 (32.7%)
62 (28.2%)
4.6% (-4.0, 13.1)
In this study, 459 of 518 (88.6%) patients had oropharyngeal candidiasis in addition to esophageal candidiasis at baseline. At the end of treatment, 192/230 (83.5%) MYCAMINE-treated patients and 188/229 (82.1%) of fluconazole-treated patients experienced resolution of signs and symptoms of oropharyngeal candidiasis. Of these, 32.3% in the MYCAMINE group, and 18.1% in the fluconazole group (treatment difference = 14.2%; 95% confidence interval [5.6, 22.8]) had symptomatic relapse at 2 weeks post-treatment. Relapse included patients who died or were lost to follow-up, and those who received systemic antifungal therapy during the post-treatment period. Cumulative relapse at 4 weeks post-treatment was 52.1% in the MYCAMINE group and 39.4% in the fluconazole group (treatment difference 12.7%, 95% confidence interval [2.8, 22.7]).
14.3 Prophylaxis of Candida Infections in Hematopoietic Stem Cell Transplant Recipients
In a randomized, double-blind study, MYCAMINE (50 mg IV once daily) was compared to fluconazole (400 mg IV once daily) in 882 [adult (791) and pediatric (91)] patients undergoing an autologous or syngeneic (46%) or allogeneic (54%) stem cell transplant. All pediatric patients, except 2 per group, received allogeneic transplants. The status of the patients’ underlying malignancy at the time of randomization was: 365 (41%) patients with active disease, 326 (37%) patients in remission, and 195 (22%) patients in relapse. The more common baseline underlying diseases in the 476 allogeneic transplant recipients were: chronic myelogenous leukemia (22%), acute myelogenous leukemia (21%), acute lymphocytic leukemia (13%), and non-Hodgkin’s lymphoma (13%). In the 404 autologous and syngeneic transplant recipients the more common baseline underlying diseases were: multiple myeloma (37.1%), non-Hodgkin’s lymphoma (36.4%), and Hodgkin's disease (15.6%). During the study, 198 of 882 (22.4%) transplant recipients had proven graft-versus-host disease; and 475 of 882 (53.9%) recipients received immunosuppressive medications for treatment or prophylaxis of graft-versus-host disease.
Study drug was continued until the patient had neutrophil recovery to an absolute neutrophil count (ANC) of 500 cells/mm3 or greater or up to a maximum of 42 days after transplant. The average duration of drug administration was 18 days (range 1 to 51 days). Duration of therapy was slightly longer in the pediatric patients who received MYCAMINE (median duration 22 days) compared to the adult patients who received MYCAMINE (median duration 18 days).
Successful prophylaxis was defined as the absence of a proven, probable, or suspected systemic fungal infection through the end of therapy (usually 18 days), and the absence of a proven or probable systemic fungal infection through the end of the 4-week post-therapy period. A suspected systemic fungal infection was diagnosed in patients with neutropenia (ANC less than 500 cells/mm3); persistent or recurrent fever (while ANC less than 500 cells/mm3) of no known etiology; and failure to respond to at least 96 hours of broad spectrum antibacterial therapy. A persistent fever was defined as four consecutive days of fever greater than 38ºC. A recurrent fever was defined as having at least one day with temperatures 38.5ºC or higher after having at least one prior temperature higher than 38ºC; or having two days of temperatures higher than 38ºC after having at least one prior temperature higher than 38ºC. Transplant recipients who died or were lost to follow-up during the study were considered failures of prophylactic therapy.
Successful prophylaxis was documented in 80.7% of adult and pediatric MYCAMINE recipients, and in 73.7% of adult and pediatric patients who received fluconazole (7.0% difference [95% CI = 1.5, 12.5]), as shown in Table 12, along with other study endpoints. The use of systemic antifungal therapy post-treatment was 42% in both groups.
The number of proven breakthrough Candida infections was 4 in the MYCAMINE and 2 in the fluconazole group.
The efficacy of MYCAMINE against infections caused by fungi other than Candida has not been established.
Table 12. Results from Clinical Study of Prophylaxis of Candida Infections in Hematopoietic Stem Cell Transplant Recipients Outcome of Prophylaxis
MYCAMINE
50 mg/day
(n = 425)
Fluconazole
400 mg/day
(n = 457)
Success*
343 (80.7%)
337 (73.7%)
Failure:
82 (19.3%)
120 (26.3%)
All Deaths†
Proven/probable fungal infection prior to death
18 (4.2%)
1 (0.2%)
26 (5.7%)
3 (0.7%)
Proven/probable fungal infection (not resulting in death)†
6 (1.4%)
8 (1.8%)
Suspected fungal infection‡
53 (12.5%)
83 (18.2%)
Lost to follow-up
5 (1.2%)
3 (0.7%)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
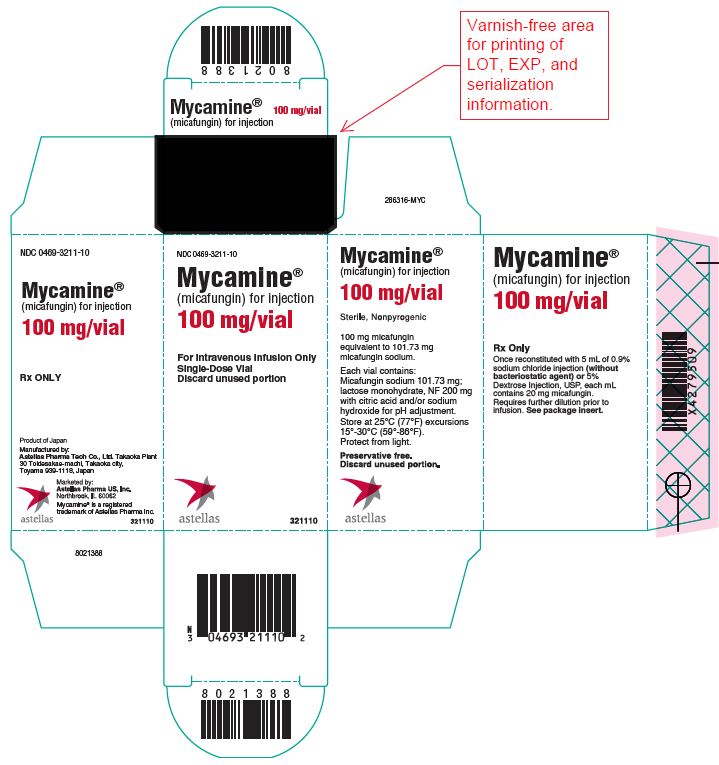
MYCAMINE is supplied as a sterile, white lyophilized powder for reconstitution for intravenous infusion, and is available in the following packaging configurations:
- •
- cartons of 10 individually packaged 50 mg single-dose vials, coated with a light protective film and sealed with a blue flip-off cap. (NDC 0469-3250-10)
- •
- cartons of 10 individually packaged 100 mg single-dose vials, coated with a light protective film and sealed with a red flip-off cap. (NDC 0469-3211-10)
Storage
Unopened vials of lyophilized material must be stored at room temperature, 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Store the reconstituted product at 25°C (77°F) [see Dosage and Administration (2.4)]
Store the diluted solution at 25°C (77°F) [see Dosage and Administration (2.4)]
Protect from light.
-
17 PATIENT COUNSELING INFORMATION
Hypersensitivity
Inform patients about the serious adverse effects of MYCAMINE including hypersensitivity reactions
e.g., anaphylaxis and anaphylactoid reactions including shock.Hepatic
Inform patients about the serious adverse effects of MYCAMINE including hepatic effects e.g., abnormal liver tests, hepatic impairment, hepatitis or worsening hepatic failure.
Hematologic
Inform patients about the serious adverse effects of MYCAMINE including hematological effects e.g., acute intravascular hemolysis, hemolytic anemia and hemoglobinuria.
Renal
Inform patients about the serious adverse effects of MYCAMINE including renal effects e.g., elevations in BUN and creatinine, renal impairment or acute renal failure.
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk of MYCAMINE to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy.
Concomitant Medications
Instruct patients to inform their healthcare provider of any other medications they are currently taking with MYCAMINE, including over-the-counter medications.
Manufactured by:
Astellas Pharma Tech Co., Ltd. Takaoka Plant
30 Toidesakae-machi, Takaoka city, Toyama 939-1118, JapanMarketed by:
Astellas Pharma US, Inc.
Northbrook, IL 60062 USAMYCAMINE® is a registered trademark of Astellas Pharma Inc.
286316-MYC
- PRINCIPAL DISPLAY PANEL – 50 mg/vial
- PRINCIPAL DISPLAY PANEL – 100 mg/vial
-
INGREDIENTS AND APPEARANCE
MYCAMINE
micafungin sodium injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0469-3250 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MICAFUNGIN SODIUM (UNII: IS1UP79R56) (MICAFUNGIN - UNII:R10H71BSWG) MICAFUNGIN SODIUM 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0469-3250-10 10 in 1 CARTON 03/16/2005 1 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021506 03/16/2005 MYCAMINE
micafungin sodium injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0469-3211 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MICAFUNGIN SODIUM (UNII: IS1UP79R56) (MICAFUNGIN - UNII:R10H71BSWG) MICAFUNGIN SODIUM 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0469-3211-10 10 in 1 CARTON 06/27/2006 1 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021506 06/27/2006 Labeler - Astellas Pharma US, Inc. (605764828)