Label: RESINOL- petrolatum and resorcinol ointment
-
NDC Code(s):
67492-105-11,
67492-105-12,
67492-105-13,
67492-105-14, view more67492-105-15
- Packager: ResiCal, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Uses
- Warnings
- Directions
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
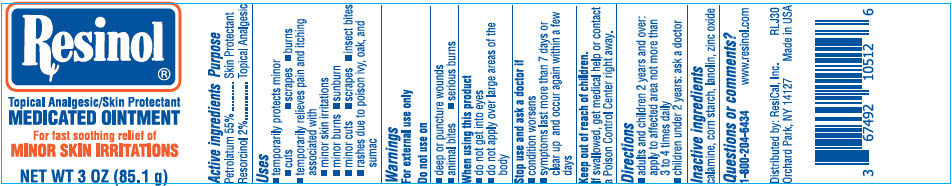
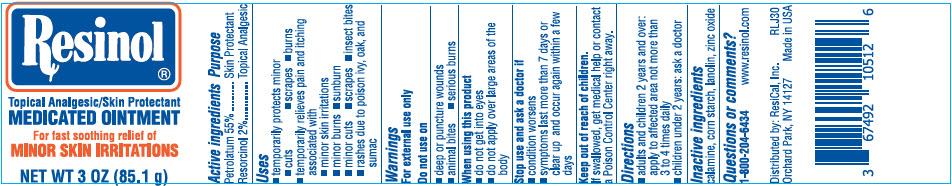
- PRINCIPAL DISPLAY PANEL - 85.1 g Jar Label
-
INGREDIENTS AND APPEARANCE
RESINOL
petrolatum and resorcinol ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67492-105 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 55 g in 100 g RESORCINOL (UNII: YUL4LO94HK) (RESORCINOL - UNII:YUL4LO94HK) RESORCINOL 2 g in 100 g Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) LANOLIN (UNII: 7EV65EAW6H) ZINC OXIDE (UNII: SOI2LOH54Z) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67492-105-12 93.6 g in 1 JAR; Type 0: Not a Combination Product 06/29/2002 12/19/2019 2 NDC:67492-105-11 35.4 g in 1 JAR; Type 0: Not a Combination Product 06/29/2002 11/01/2023 3 NDC:67492-105-13 25.5 g in 1 JAR; Type 0: Not a Combination Product 06/29/2002 4 NDC:67492-105-14 85.1 g in 1 JAR; Type 0: Not a Combination Product 06/29/2002 5 NDC:67492-105-15 50 g in 1 TUBE; Type 0: Not a Combination Product 09/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M017 06/29/2002 Labeler - ResiCal, Inc. (927081369) Establishment Name Address ID/FEI Business Operations Aurora Labs LLC. 176110547 MANUFACTURE(67492-105)