Label: FERTAGYL- gonadorelin injection
- NDC Code(s): 57926-477-07, 57926-477-67
- Packager: Merck Sharp & Dohme Corp.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated June 15, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
189979 R9
For treatment of cystic ovaries in dairy cattle
For use with Estrumate (cloprostenol injection) to synchronize estrous cycles to allow for fixed time artificial insemination (FTAI) in lactating dairy cows
For use with cloprostenol sodium to synchronize estrous cycles to allow for FTAI in beef cows
- PRECAUTIONS
-
DESCRIPTION
DESCRIPTION:
Fertagyl is a sterile solution containing 43 mcg/mL of gonadorelin (GnRH: as gonadorelin acetate) suitable for intramuscular or intravenous administration according to the indication.
Gonadorelin is a decapeptide composed of the sequence of amino acids –
5-oxoPro-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2
a molecular weight of 1182.32 and empirical formula C55H75N17O13.
Each mL of Fertagyl contains:
Gonadorelin (as gonadorelin acetate) 43 mcg Benzyl Alcohol 9 mg Sodium Chloride 7.47 mg Water for Injection, USP q.s. pH adjusted with sodium phosphate (monobasic and dibasic).
Gonadorelin is the hypothalamic releasing factor responsible for the release of gonadotropins (e.g., luteinizing hormone [LH], follicle stimulating hormone [FSH]) from the anterior pituitary.
Synthetic gonadorelin is physiologically and chemically identical to the endogenous bovine hypothalamic releasing factor.
-
INDICATIONS & USAGE
INDICATIONS FOR USE:
Cystic Ovaries
Fertagyl is indicated for the treatment of ovarian follicular cysts in dairy cattle. Ovarian cysts are nonovulated follicles with incomplete luteinization which result in nymphomania or irregular estrus.
Historically, cystic ovaries have responded to an exogenous source of LH such as human chorionic gonadotropin.
Fertagyl initiates release of endogenous LH to cause ovulation and luteinization.
Reproductive Synchrony
Fertagyl is indicated for use with Estrumate (cloprostenol injection) to synchronize estrous cycles to allow for fixed time artificial insemination (FTAI) in lactating dairy cows.
Fertagyl is indicated for use with cloprostenol sodium to synchronize estrous cycles to allow for FTAI in beef cows.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION:
Cystic Ovaries
The intravenous or intramuscular dosage of Fertagyl is 86 mcg gonadorelin (2 mL) per cow.
Reproductive Synchrony
For lactating dairy cows, the intramuscular dosage of Fertagyl is 86 mcg gonadorelin (2 mL) per cow, used in reproductive synchrony programs similar to the following:
- Administer the first Fertagyl injection (2 mL) on Day 0.
- Administer 2 mL of Estrumate (500 mcg cloprostenol, as cloprostenol sodium) by intramuscular injection 6 to 8 days after the first Fertagyl injection.
- Administer the second Fertagyl injection (2 mL) 30 to 72 hours after the Estrumate injection.
- Perform FTAI 8 to 24 hours after the second Fertagyl injection, or inseminate cows on detected estrus using standard herd practices.
For beef cows, the intramuscular dosage of Fertagyl is 86 mcg gonadorelin (2 mL) per cow, used in reproductive synchrony programs similar to the following:
- Administer the first Fertagyl injection (2 mL) on Day 0.
- Administer 500 mcg cloprostenol (as cloprostenol sodium) by intramuscular injection 6 to 8 days after the first Fertagyl injection.
- Administer the second Fertagyl injection (2 mL) 30 to 72 hours after the cloprostenol sodium injection.
- Perform FTAI 0 to 24 hours after the second Fertagyl injection, or inseminate cows on detected estrus using standard herd practices.
- WARNINGS
-
SPL UNCLASSIFIED SECTION
WITHDRAWAL PERIODS:
No withdrawal period or milk discard time is required when used according to the labeling.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Intervet at 1-800-211-3573. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or http://www.fda.gov/reportanimalae.
-
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
PHARMACOLOGY AND TOXICOLOGY:
Endogenous gonadorelin is synthesized and/or released from the hypothalamus during various stages of the bovine estrous cycle following appropriate neurogenic stimuli. It passes via the hypophyseal portal vessels, to the anterior pituitary to effect the release of gonadotropins (e.g. LH, FSH).
Synthetic gonadorelin administered intravenously or intramuscularly also causes the release of endogenous LH or FSH from the anterior pituitary.
Gonadorelin acetate has been shown to be safe. The LD50 for mice and rats is greater than 60 mg/kg, and for dogs, greater than 600 mcg/kg, respectively. No adverse effects were noted among rats or dogs administered 120 mcg/kg/day or 72 mcg/kg/day intravenously for 15 days.
It had no adverse effects on heart rate, blood pressure, or EKG to unanesthetized dogs at 60 mcg/kg. In anesthetized dogs it did not produce depression of myocardial or system hemodynamics or adversely affect coronary oxygen supply or myocardial oxygen requirements.
The intravenous administration of 60 mcg/kg/day gonadorelin acetate to pregnant rats and rabbits during organogenesis did not cause embryotoxic or teratogenic effects.
Further, gonadorelin acetate did not cause irritation at the site of intramuscular administration in dogs with a dose of 72 mcg/kg/day administered for seven (7) days.
-
SPL UNCLASSIFIED SECTION
TARGET ANIMAL SAFETY:
In addition to the animal safety information presented in the PHARMACOLOGY AND TOXICOLOGY section, the safety of gonadorelin was established through the review and evaluation of the extensive published literature available for the use of gonadorelin-containing products.
The intramuscular administration of 860 mcg gonadorelin (as gonadorelin acetate) on five (5) consecutive days to normally cycling dairy cattle had no effect on hematology or clinical chemistries. In field studies evaluating the effectiveness of gonadorelin for the treatment of ovarian follicular cysts, the incidence of health abnormalities was not significantly greater in cows administered gonadorelin than cows administered a placebo injection.
The target animal safety of, and injection site reactions to, Fertagyl when used with Estrumate (cloprostenol injection) were evaluated during the conduct of effectiveness field studies in lactating dairy cows. The incidence of health abnormalities was not significantly greater in cows administered Fertagyl than cows administered a placebo injection.
The target animal safety of, and injection site reactions to, gonadorelin when used with cloprostenol sodium were evaluated during the conduct of effectiveness field studies in beef cows. The incidence of health abnormalities was not significantly greater in cows administered gonadorelin than cows administered a placebo injection.
-
SPL UNCLASSIFIED SECTION
EFFECTIVENESS:
The use of gonadorelin for treatment of ovarian follicular cysts in dairy cattle was demonstrated to be effective with a treatment dose of 86 mcg gonadorelin (as gonadorelin acetate).
The effectiveness of Fertagyl for use with Estrumate (cloprostenol injection) to synchronize estrous cycles to allow for FTAI in lactating dairy cows was demonstrated in a field study at six different locations in the U.S. A total of 758 healthy, non-pregnant, primiparous or multiparous lactating dairy cows within 50-120 days postpartum were enrolled in the study. A total of 377 cows were administered Fertagyl (2 mL; 86 mcg gonadorelin as the acetate salt) and 381 cows were administered an equivalent volume of saline as an intramuscular injection twice in the following regimen:
Day 0: 2 mL Fertagyl or saline
Day 7: 2 mL Estrumate (cloprostenol injection)
Day 9: 2 mL Fertagyl or salineFixed time Al was performed on Day 10, 16 ± 8 hours after the Day 9 injection. Cows were evaluated for pregnancy on Day 45 ± 5 days by trans-rectal ultrasound or rectal palpation. Pregnancy rate to FTAI was significantly higher (P=0.0051) in cows treated with Fertagyl (33.4%) than the pregnancy rate to FTAI to cows treated with saline (17.8%).
The effectiveness of gonadorelin for use with cloprostenol sodium to synchronize estrous cycles to allow for FTAI in beef cows was demonstrated in a field study at 10 different locations in the U.S. A total of 706 healthy, non-pregnant, primiparous or multiparous beef cows within 40-150 days postpartum were enrolled in the study. A total of 364 cows were administered gonadorelin (1 mL; 100 mcg gonadorelin as the acetate salt) and 342 cows were administered an equivalent volume of water for injection as an intramuscular injection twice in the following regimen:
Day 0: 100 mcg gonadorelin (as the acetate salt) or sterile water for injection
Day 7: 500 mcg cloprostenol (as cloprostenol sodium)
Day 9: 100 mcg gonadorelin (as the acetate salt) or sterile water for injectionFixed time AI was performed immediately after the Day 9 injection. Cows were evaluated for pregnancy on Day 55±5 days by trans-rectal ultrasound. Pregnancy rate to FTAI was significantly higher (P=0.0006) in cows treated with gonadorelin (21.7%) than the pregnancy rate to FTAI in cows treated with water (7.4%).
The effectiveness of a 2-mL dose of gonadorelin delivering 86 mcg gonadorelin (as gonadorelin acetate) for use with cloprostenol sodium to synchronize estrous cycles to allow for FTAI in lactating dairy cows and beef cows was also demonstrated through references to scientific literature.
-
HOW SUPPLIED
HOW SUPPLIED:
Fertagyl is available in a concentration of 43 mcg/mL gonadorelin (as gonadorelin acetate) pH adjusted with sodium phosphate (monobasic and dibasic).
Fertagyl is supplied in multi-dose vials containing 20 mL and 100 mL of sterile solution.
STORAGE, HANDLING, AND DISPOSAL: Keep refrigerated: 2°-8°C (36°-46°F).
20 mL vial: Use within 28 days of first puncture.
100 mL vial: Use within 28 days of first puncture and puncture a maximum of 10 times when using an 18 gauge needle. When using a draw-off spike or needle with bore diameter larger than 18 gauge, discard any product remaining in the vial immediately after use.
-
SPL UNCLASSIFIED SECTION
Approved by FDA under ANADA # 200-134
Manufactured for:
Intervet Inc. (d/b/a Merck Animal Health)
Madison, NJ 07940
Gonadorelin (active ingred.) made in the Netherlands. Formulated in Germany.Copyright©2020 Intervet Inc. (d/b/a Merck Animal Health), a subsidiary of Merck and Co., Inc.
All rights reserved.Rev. 02/2020
-
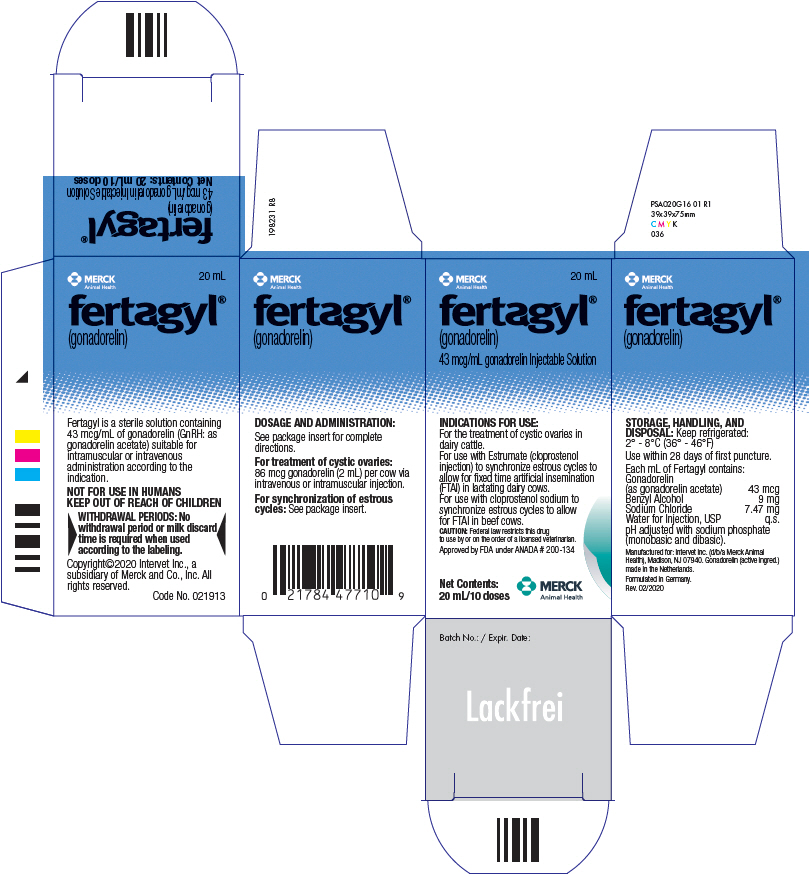
PRINCIPAL DISPLAY PANEL - 20 mL Vial Carton
MERCK
Animal Health20 mL
fertagyl®
(gonadorelin)
43 mcg/mL gonadorelin Injectable SolutionINDICATIONS FOR USE:
For the treatment of cystic ovaries in
dairy cattle.
For use with Estrumate (cloprostenol
injection) to synchronize estrous cycles to
allow for fixed time artificial insemination
(FTAI) in lactating dairy cows.
For use with cloprostenol sodium to
synchronize estrous cycles to allow
for FTAI in beef cows.CAUTION: Federal law restricts this drug
to use by or on the order of a licensed veterinarian.Approved by FDA under ANADA # 200-134
Net Contents:
20 mL/10 dosesMERCK
Animal Health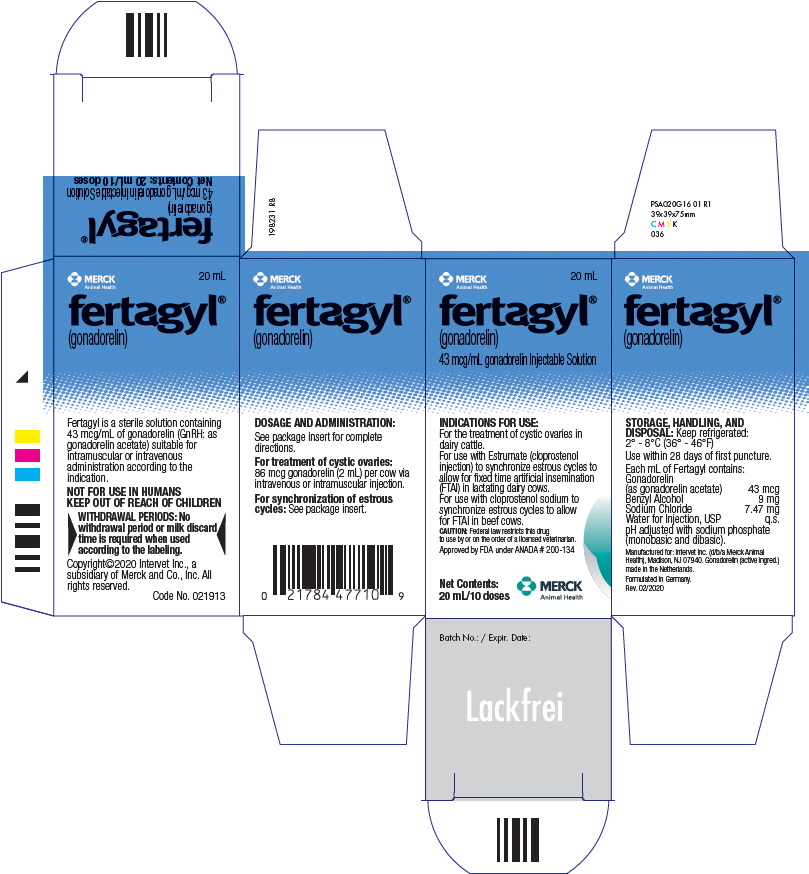
-
INGREDIENTS AND APPEARANCE
FERTAGYL
gonadorelin injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:57926-477 Route of Administration INTRAMUSCULAR, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GONADORELIN (UNII: 9O7312W37G) (GONADORELIN - UNII:9O7312W37G) GONADORELIN 43 ug in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) Sodium Phosphate, Monobasic, Dihydrate (UNII: 5QWK665956) Benzyl Alcohol (UNII: LKG8494WBH) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57926-477-07 1 in 1 CARTON 1 20 mL in 1 VIAL, MULTI-DOSE 2 NDC:57926-477-67 1 in 1 CARTON 2 100 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200134 06/17/1996 Labeler - Merck Sharp & Dohme Corp. (001317601) Establishment Name Address ID/FEI Business Operations Intervet International GMBH 328855635 MANUFACTURE Establishment Name Address ID/FEI Business Operations Aspen Oss B.V. 491017488 API MANUFACTURE