Label: ZOLADEX- goserelin implant
- NDC Code(s): 70720-951-30
- Packager: TerSera Therapeutics LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 25, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ZOLADEX safely and effectively.
See full prescribing information for ZOLADEX.
ZOLADEX® (goserelin implant) 10.8 mg
Initial U.S. Approval: 1996INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Implant 10.8 mg (3)
WARNINGS AND PRECAUTIONS
- Tumor Flare Phenomenon: Transient worsening of tumor symptoms may occur during the first few weeks of treatment with ZOLADEX, which may include ureteral obstruction and spinal cord compression. Monitor patients at risk for complications of tumor flare (5.1, 6.1)
- Hypersensitivity: Systemic hypersensitivity has been reported in patients receiving goserelin implants (4.1, 5.2)
- Hyperglycemia and Diabetes: Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH analogs. Monitor blood glucose level and manage according to current clinical practice (5.3)
- Cardiovascular Diseases: Increased risk of myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH analogs in men. Monitor for cardiovascular disease and manage according to current clinical practice (5.4)
- Effect on QT/QTc Interval: Androgen deprivation therapy may prolong the QT interval. Consider risks and benefits (5.5)
- Injection Site Injury: Injection site injury and vascular injury have been reported during administration (5.6)
ADVERSE REACTIONS
The most common, clinically significant adverse reactions occurring in >10% of men: hot flashes, sexual dysfunction, decreased erections and lower urinary tract symptoms (6)
Tumor flare can occur on the initiation of ZOLADEX therapy (5.1, 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact TerSera Therapeutics at 1-844-334-4035 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- None
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Stage B2-C Prostatic Carcinoma
1.2 Prostatic Carcinoma
2 DOSAGE AND ADMINISTRATION
2.1 Stage B2-C Prostatic Carcinoma
2.2 Prostatic Carcinoma
2.3 Renal or Hepatic Impairment
2.4 Women
2.5 Administration Technique
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
4.2 Pregnancy
5 WARNINGS AND PRECAUTIONS
5.1 Tumor Flare Phenomenon
5.2 Hypersensitivity
5.3 Hyperglycemia and Diabetes
5.4 Cardiovascular Diseases
5.5 Effect on QT/QTc Interval
5.6 Injection Site Injury
6 ADVERSE REACTIONS
6.1 Clinical Trials
6.2 Stage B2-C Prostatic Carcinoma
6.3 Prostatic Carcinoma
6.4 Changes in Laboratory Values During Treatment
6.5 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drug/Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Insufficiency
8.7 Hepatic Insufficiency
8.8 Body Weight
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Stage B2-C Prostatic Carcinoma
14.2 Prostatic Carcinoma
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Males
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Stage B2-C Prostatic Carcinoma
ZOLADEX is indicated for use in combination with flutamide for the management of locally confined Stage T2b-T4 (Stage B2-C) carcinoma of the prostate. Treatment with ZOLADEX and flutamide should start 8 weeks prior to initiating radiation therapy and continue during radiation therapy [see Dosage and Administration (2.1) and Clinical Studies (14.1)].
1.2 Prostatic Carcinoma
ZOLADEX is indicated in the palliative treatment of advanced carcinoma of the prostate [see Dosage and Administration (2.2) and Clinical Studies (14.2)].
In controlled studies of patients with advanced prostatic cancer comparing ZOLADEX 3.6 mg to orchiectomy, the long-term endocrine responses and objective responses were similar between the two treatment arms. Additionally, duration of survival was similar between the two treatment arms in a major comparative trial.
In controlled studies of patients with advanced prostatic cancer, ZOLADEX 10.8 mg implant produced pharmacodynamically similar effect in terms of suppression of serum testosterone to that achieved with ZOLADEX 3.6 mg implant. Clinical outcome similar to that produced with the use of the ZOLADEX 3.6 mg implant administered every 28 days is predicted with the ZOLADEX 10.8 mg implant administered every 12 weeks.
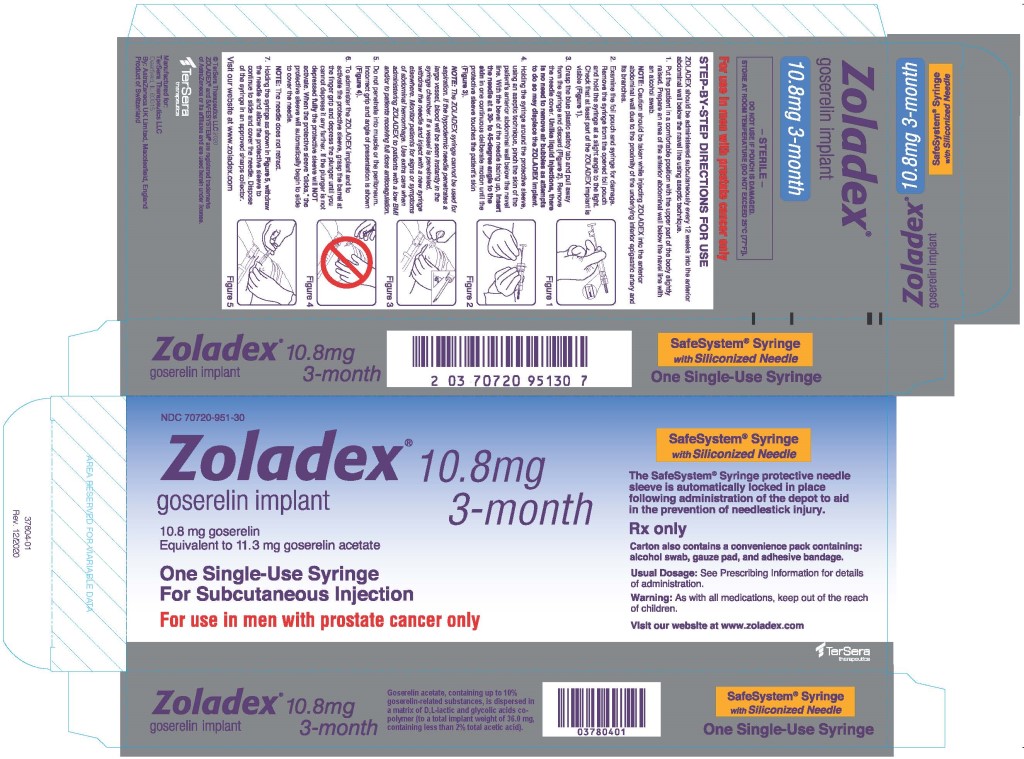
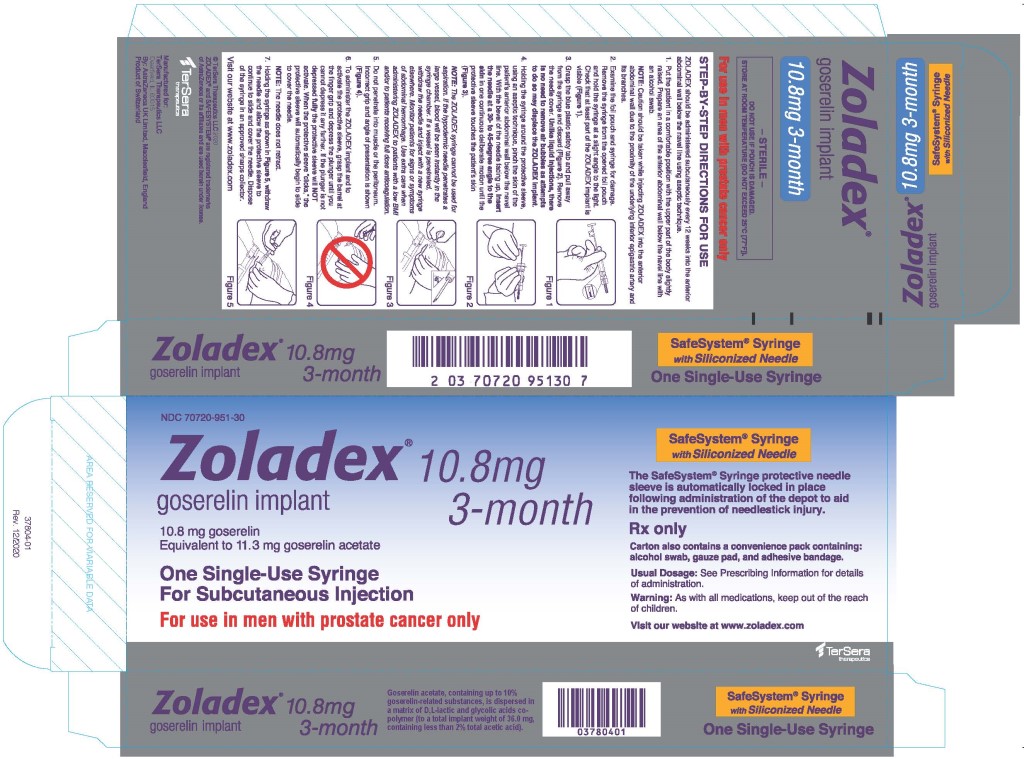
The automatic safety feature of the syringe aids in the prevention of needlestick injury.
-
2 DOSAGE AND ADMINISTRATION
ZOLADEX, at a dose of 10.8 mg, should be administered subcutaneously every 12 weeks into the anterior abdominal wall below the navel line using an aseptic technique under the supervision of a physician [see Dosage and Administration (2.5)].
While a delay of a few days is permissible, every effort should be made to adhere to the 12-week schedule.
2.1 Stage B2-C Prostatic Carcinoma
When ZOLADEX is given in combination with radiotherapy and flutamide for patients with Stage T2b-T4 (Stage B2-C) prostatic carcinoma, treatment should be started 8 weeks prior to initiating radiotherapy and should continue during radiation therapy. A treatment regimen using one ZOLADEX 3.6 mg depot, followed in 28 days by one ZOLADEX 10.8 mg depot, should be administered.
2.2 Prostatic Carcinoma
For the management of advanced prostate cancer, ZOLADEX is intended for long-term administration unless clinically inappropriate.
2.3 Renal or Hepatic Impairment
No dosage adjustment is necessary for patients with renal or hepatic impairment.
2.4 Women
ZOLADEX 10.8 mg implant is not indicated in women as the data are insufficient to support reliable suppression of serum estradiol. For female patients requiring treatment with goserelin, refer to prescribing information for ZOLADEX 3.6 mg implant.
2.5 Administration Technique
The proper method of administration of ZOLADEX is described in the instructions that follow.
- Put the patient in a comfortable position with the upper part of the body slightly raised. Prepare an area of the anterior abdominal wall below the navel line with an alcohol swab.
NOTE: Caution should be taken while injecting ZOLADEX into the anterior abdominal wall due to the proximity of underlying inferior epigastric artery and its branches. - Examine the foil pouch and syringe for damage. Remove the syringe from the opened foil pouch and hold the syringe at a slight angle to the light. Check that at least part of the ZOLADEX implant is visible.
- Grasp the blue plastic safety tab and pull away from the syringe, and discard. Remove needle cover. Unlike liquid injections, there is no need to remove air bubbles as attempts to do so may displace the ZOLADEX implant.
- Holding the syringe around the protective sleeve, using an aseptic technique, pinch the skin of the patient's anterior abdominal wall below the navel line. With the bevel of the needle facing up, insert the needle at a 30 to 45 degree angle to the skin in one continuous deliberate motion until the protective sleeve touches the patient's skin.
NOTE: The ZOLADEX syringe cannot be used for aspiration. If the hypodermic needle penetrates a large vessel, blood will be seen instantly in the syringe chamber. If a vessel is penetrated, withdraw the needle and inject with a new syringe elsewhere. Monitor patients for signs or symptoms of abdominal hemorrhage. Use extra care when administering ZOLADEX to patients with a low BMI and/or to patients receiving full dose anticoagulation [see Warnings and Precautions (5.6)]. - Do not penetrate into muscle or peritoneum.
- To administer the ZOLADEX implant and to activate the protective sleeve, grasp the barrel at the finger grip and depress the plunger until you cannot depress it any further. If the plunger is not depressed fully, the protective sleeve will NOT activate. When the protective sleeve ‘clicks’, the protective sleeve will automatically begin to slide to cover the needle.
NOTE: The needle does not retract. - Withdraw the needle and allow protective sleeve to slide and cover needle. Dispose of the syringe in an approved sharps collector.
NOTE: In the unlikely event of the need to surgically remove ZOLADEX, it may be localized by ultrasound.
- Put the patient in a comfortable position with the upper part of the body slightly raised. Prepare an area of the anterior abdominal wall below the navel line with an alcohol swab.
-
3 DOSAGE FORMS AND STRENGTHS
ZOLADEX 10.8 mg implant is supplied as a sterile and totally biodegradable D,L-lactic and glycolic acids copolymer impregnated with 11.3 mg goserelin acetate equivalent to 10.8 mg of goserelin in a disposable syringe device fitted with a 14-gauge x 36 +/- 0.5 mm hypodermic siliconized needle with protective needle sleeve [SafeSystem® Syringe] (NDC 70720-951-30).
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity
Anaphylactic reactions to ZOLADEX have been reported in the medical literature. ZOLADEX is contraindicated in those patients who have a known hypersensitivity to GnRH, GnRH agonist analogues or any of the components in ZOLADEX [see Warnings and Precautions (5.2)].
4.2 Pregnancy
Expected hormonal changes that occur with ZOLADEX treatment increase the risk for pregnancy loss. ZOLADEX may cause fetal harm when administered to a pregnant woman. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus [see Use in Specific Populations (8.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Tumor Flare Phenomenon
Initially, ZOLADEX, like other GnRH agonists, causes transient increases in serum levels of testosterone. Transient worsening of symptoms, or the occurrence of additional signs and symptoms of prostatic cancer, may occasionally develop during the first few weeks of ZOLADEX treatment. A small number of patients may experience a temporary increase in bone pain, which can be managed symptomatically. As with other GnRH agonists, isolated cases of ureteral obstruction and spinal cord compression have been observed. If spinal cord compression or renal impairment secondary to ureteral obstruction develops, standard treatment of these complications should be instituted, and in extreme cases an immediate orchiectomy [see Adverse Reactions (6.1) and Patient Counseling Information (17.1)].
5.2 Hypersensitivity
Hypersensitivity, antibody formation and acute anaphylactic reactions have been reported with GnRH agonist analogues [see Contraindications (4.1)].
Of 115 women worldwide treated with ZOLADEX and tested for development of binding to goserelin following treatment with ZOLADEX, one patient showed low-titer binding to goserelin. On further testing of this patient's plasma obtained following treatment, her goserelin binding component was found not to be precipitated with rabbit antihuman immunoglobulin polyvalent sera. These findings suggest the possibility of antibody formation.
5.3 Hyperglycemia and Diabetes
Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH agonists. Hyperglycemia may represent development of diabetes mellitus or worsening of glycemic control in patients with diabetes. Monitor blood glucose and/or glycosylated hemoglobin (HbA1c) periodically in patients receiving a GnRH agonist and manage with current practice for treatment of hyperglycemia or diabetes [see Patient Counseling Information (17.1)].
5.4 Cardiovascular Diseases
Increased risk of developing myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH agonists in men. The risk appears low based on the reported odds ratios, and should be evaluated carefully along with cardiovascular risk factors when determining a treatment for patients with prostate cancer. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice [see Patient Counseling Information (17.1)].
5.5 Effect on QT/QTc Interval
Androgen deprivation therapy may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, frequent electrolyte abnormalities, and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes.
5.6 Injection Site Injury
Injection site injury and vascular injury including pain, hematoma, hemorrhage and hemorrhagic shock, requiring blood transfusions and surgical intervention, have been reported with ZOLADEX. Extra care should be taken when administering ZOLADEX to patients with a low BMI and/or to patients receiving full anticoagulation [see Dosage and Administration (2.5) and Patient Counseling Information (17.1)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials
ZOLADEX has been found to be generally well tolerated in clinical trials. Adverse reactions reported in these trials were rarely severe enough to result in the patients' withdrawal from ZOLADEX treatment. As seen with other hormonal therapies, the most commonly observed adverse events during ZOLADEX therapy were due to the expected physiological effects from decreased testosterone levels. These included hot flashes, sexual dysfunction and decreased erections.
Tumor Flare Phenomenon: Initially, ZOLADEX, like other GnRH agonists, causes transient increases in serum levels of testosterone. A small percentage of patients experienced a temporary worsening of signs and symptoms, usually manifested by an increase in cancer-related pain which was managed symptomatically. Isolated cases of exacerbation of disease symptoms, either ureteral obstruction or spinal cord compression, occurred at similar rates in controlled clinical trials with both ZOLADEX and orchiectomy. The relationship of these events to therapy is uncertain [see Warnings and Precautions (5.1) and Patient Counseling Information (17.1)].
6.2 Stage B2-C Prostatic Carcinoma
Treatment with ZOLADEX and flutamide did not add substantially to the toxicity of radiation treatment alone. The following adverse experiences were reported during a multicenter clinical trial comparing ZOLADEX + flutamide + radiation versus radiation alone. The most frequently reported (greater than 5%) adverse experiences are listed below:
Table 1 ADVERSE EVENTS DURING ACUTE RADIATION THERAPY (within first 90 days of radiation therapy) (n=231)
flutamide +
ZOLADEX + Radiation
% All(n=235)
Radiation Only
% AllRectum/Large Bowel 80 76 Bladder 58 60 Skin 37 37 Table 2 ADVERSE EVENTS DURING LATE RADIATION PHASE (after 90 days of radiation therapy) (n=231)
flutamide + ZOLADEX + Radiation
% All(n=235)
Radiation Only
% AllDiarrhea 36 40 Cystitis 16 16 Rectal Bleeding 14 20 Proctitis 8 8 Hematuria 7 12 Additional adverse event data was collected for the combination therapy with radiation group over both the hormonal treatment and hormonal treatment plus radiation phases of the study. Adverse experiences occurring in more than 5% of patients in this group, over both parts of the study, were hot flashes (46%), diarrhea (40%), nausea (9%), and skin rash (8%).
6.3 Prostatic Carcinoma
Two controlled clinical trials using ZOLADEX 10.8 mg versus ZOLADEX 3.6 mg were conducted. During a comparative phase, patients were randomized to receive either a single 10.8 mg implant or three consecutive 3.6 mg implants every 4 weeks over weeks 0-12. During this phase, the only adverse event reported in greater than 5% of patients was hot flashes, with an incidence of 47% in the ZOLADEX 10.8 mg group and 48% in the ZOLADEX 3.6 mg group.
From weeks 12-48 all patients were treated with a 10.8 mg implant every 12 weeks. During this noncomparative phase, the following adverse events were reported in greater than 5% of patients:
Table 3 ADVERSE EVENTS WERE REPORTED IN GREATER THAN 5% OF PATIENTS ADVERSE EVENTS ZOLADEX 10.8 mg
(n=157)
%Hot Flashes 64 Pain (general) 14 Gynecomastia 8 Pelvic Pain 6 Bone Pain 6 Asthenia 5 The following adverse events were reported in greater than 1%, but less than 5% of patients treated with ZOLADEX 10.8 mg implant every 12 weeks. Some of these are commonly reported in elderly patients.
WHOLE BODY – Abdominal pain, Back pain, Flu syndrome, Headache, Sepsis, Aggravation reaction
CARDIOVASCULAR – Angina pectoris, Cerebral ischemia, Cerebrovascular accident, Heart failure, Pulmonary embolus, Varicose veins
DIGESTIVE – Diarrhea, Hematemesis
ENDOCRINE – Diabetes mellitus
HEMATOLOGIC – Anemia
METABOLIC – Peripheral edema
NERVOUS SYSTEM – Dizziness, Paresthesia, Urinary retention
RESPIRATORY – Cough increased, Dyspnea, Pneumonia
SKIN – Herpes simplex, Pruritus
UROGENITAL – Bladder neoplasm, Breast pain, Hematuria, Impotence, Urinary frequency, Urinary incontinence, Urinary tract disorder, Urinary tract infection, Urination impaired
The following adverse events not already listed above were reported in patients receiving ZOLADEX 3.6 mg in other clinical trials. Inclusion does not necessarily represent a causal relationship to ZOLADEX 10.8 mg.
WHOLE BODY: Allergic reaction, Chills, Fever, Infection, Injection site reaction, Lethargy, Malaise
CARDIOVASCULAR: Arrhythmia, Chest pain, Hemorrhage, Hypertension, Migraine, Myocardial infarction, Palpitations, Peripheral vascular disorder, Tachycardia
DIGESTIVE: Anorexia, Constipation, Dry mouth, Dyspepsia, Flatulence, Increased appetite, Nausea, Ulcer, Vomiting
HEMATOLOGIC: Ecchymosis
METABOLIC: Edema, Gout, Hyperglycemia, Weight increase
MUSCULOSKELETAL: Arthralgia, Hypertonia, Joint disorder, Leg cramps, Myalgia, Osteoporosis
NERVOUS SYSTEM: Anxiety, Depression, Emotional lability, Headache, Insomnia, Nervousness, Somnolence, Thinking abnormal
RESPIRATORY: Bronchitis, Chronic obstructive pulmonary disease, Epistaxis, Rhinitis, Sinusitis, Upper respiratory infection, Voice alterations
SKIN: Acne, Alopecia, Dry skin, Hair disorders, Rash, Seborrhea, Skin discoloration, Sweating
SPECIAL SENSES: Amblyopia, Dry eyes
UROGENITAL: Breast tenderness, Decreased erections, Renal insufficiency, Sexual dysfunction, Urinary obstruction
6.4 Changes in Laboratory Values During Treatment
Plasma Enzymes: Elevation of liver enzymes (AST, ALT) have been reported in female patients exposed to ZOLADEX 3.6 mg (representing less than 1% of all patients). There was no other evidence of abnormal liver function. Causality between these changes and ZOLADEX have not been established.
Lipids: In a controlled trial in females, ZOLADEX 3.6 mg implant therapy resulted in a minor, but statistically significant effect on serum lipids (i.e., increases in LDL cholesterol of 21.3 mg/dL; increases in HDL cholesterol of 2.7 mg/dL; and triglycerides increased by 8.0 mg/dL).
6.5 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ZOLADEX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypercalcemia: In patients with bone metastases.
Bone Mineral Density: Osteoporosis, decreased bone mineral density and bone fracture in men [see Patient Counseling Information (17.1)].
Changes in Blood Pressure: Hypotension and hypertension have been reported. These changes are usually transient, resolving either during continued therapy or after cessation of therapy.
Pituitary Apoplexy and Tumors: Pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) and pituitary adenoma have been diagnosed. Most of the pituitary apoplexy cases occurred within 2 weeks of the first dose, and some occurred within the first hour. In these cases, pituitary apoplexy has presented as sudden headache, vomiting, visual changes, ophthalmoplegia, altered mental status, and sometimes cardiovascular collapse. Immediate medical attention has been required. Pituitary tumors have been reported.
Acne: Usually within one month of starting treatment.
Other Adverse Reactions: Psychotic disorders, convulsions and mood swings.
-
7 DRUG INTERACTIONS
No formal drug-drug interaction studies have been performed.
No drug interaction studies with other drugs have been conducted with ZOLADEX. No confirmed interactions have been reported between ZOLADEX and other drugs.
7.1 Drug/Laboratory Test Interactions
Administration of ZOLADEX in therapeutic doses results in suppression of the pituitary-gonadal system. Because of this suppression, diagnostic tests of pituitary-gonadotropic and gonadal functions conducted during treatment may show results which are misleading.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Based on mechanism of action in humans and findings of increased pregnancy loss in animal studies, ZOLADEX may cause fetal harm when administered to a pregnant woman. Expected hormone changes that occur with ZOLADEX treatment increase the risk for pregnancy loss. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus [see Contraindications (4.2)].
ZOLADEX crosses the placenta in rats and rabbits following subcutaneous administration. Administration of goserelin to pregnant rats and rabbits during organogenesis resulted in increased preimplantation loss and increased resorptions. When pregnant rats received goserelin throughout gestation and lactation, there was a dose-related increase in umbilical hernia in offspring. In additional reproduction studies in rats, goserelin decreased fetus and pup survival. Human dose/exposure multiples could not be calculated from available animal data.
Actual animal doses: rat (≥ 2 mcg/kg/day for pregnancy loss; ≥ 10 mcg/kg/day for umbilical hernia in offspring); rabbits (> 20 mcg/kg/day).
8.3 Nursing Mothers
It is not known if goserelin is excreted in human milk. Goserelin is excreted in the milk of lactating rats. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from ZOLADEX, a decision should be made to either discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
8.5 Geriatric Use
There is no need for any dosage adjustment when administering ZOLADEX 10.8 mg to geriatric patients.
8.6 Renal Insufficiency
In clinical trials with the solution formulation of goserelin, subjects with impaired renal function (creatinine clearance < 20 mL/min) had a serum elimination half-life of 12.1 hours compared to 4.2 hours for subjects with normal renal function (creatinine clearance > 70 mL/min). However, there was no evidence for any accumulation of goserelin on multiple dosing of the ZOLADEX 10.8 mg depot to subjects with impaired renal function. There was no evidence for any increase in incidence of adverse events in renally impaired patients administered the 10.8 mg depot. These data indicate that there is no need for any dosage adjustment when administering ZOLADEX 10.8 mg to subjects with impaired renal function.
8.7 Hepatic Insufficiency
The total body clearances and serum elimination half-lives were similar between normal subjects and patients with moderate hepatic impairment (alanine transaminase < 3xULN and asparate aminotransferase < 3xULN) when treated with a 250 mcg subcutaneous formulation of goserelin. This pharmacokinetic study indicates that no dose adjustment is needed in patients with moderately impaired liver function. There is no pharmacokinetic data with goserelin in patients with severe hepatic insufficiency.
-
10 OVERDOSAGE
The pharmacologic properties of ZOLADEX and its mode of administration make accidental or intentional overdosage unlikely. There is no experience of overdosage from clinical trials. Animal studies indicate that no increased pharmacologic effect occurred at higher doses or more frequent administration. Subcutaneous doses of the drug as high as 1 mg/kg/day in rats and dogs did not produce any nonendocrine related sequelae; this dose is up to 250 times the estimated human daily dose based on the body surface area. If overdosage occurs, it should be managed symptomatically.
-
11 DESCRIPTION
ZOLADEX (goserelin implant) is a GnRH agonist. Goserelin acetate is chemically described as an acetate salt of [D-Ser(But)6,Azgly10]. Its chemical structure is pyro-Glu-His-Trp-Ser-Tyr-D-Ser(But)-Leu-Arg-Pro-Azgly-NH2 acetate [C59H84N18O14·(C2H4O2)x where x = 1 to 2.4].
Goserelin acetate is an off-white powder with a molecular weight of 1269 Daltons (free base). It is freely soluble in glacial acetic acid. It is soluble in water, 0.1M hydrochloric acid, 0.1M sodium hydroxide, dimethylformamide and dimethyl sulfoxide. Goserelin acetate is practically insoluble in acetone, chloroform and ether.
ZOLADEX 10.8 mg implant is supplied as a sterile, biodegradable product containing 11.3 mg goserelin acetate equivalent to 10.8 mg of goserelin. ZOLADEX is designed for subcutaneous implantation with continuous release over a 12-week period. Goserelin acetate, containing up to 10% goserelin-related substances, is dispersed in a matrix of D,L-lactic and glycolic acids copolymer (to a total implant weight of 36.0 mg, containing less than 2% total acetic acid) and presented as a sterile, white to cream colored 1.5 mm diameter cylinder, preloaded in a special single-use syringe with a 14-gauge x 36 +/- 0.5 mm siliconized needle with protective needle sleeve (SafeSystem® Syringe) in a sealed, light- and moisture-proof, aluminum foil laminate pouch containing a desiccant capsule.
Studies of the D,L-lactic and glycolic acids copolymer have indicated that it is completely biodegradable and has no demonstrable antigenic potential.
ZOLADEX is also supplied as a sterile, biodegradable product containing goserelin acetate equivalent to 3.6 mg of goserelin designed for administration every 28 days.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ZOLADEX is a synthetic decapeptide analogue of GnRH. ZOLADEX acts as an inhibitor of pituitary gonadotropin secretion when administered in the biodegradable formulation.
In animal and in vitro studies, administration of goserelin resulted in the regression or inhibition of growth of the hormonally sensitive dimethylbenzanthracene (DMBA)-induced rat mammary tumor and Dunning R3327 prostate tumor.
12.2 Pharmacodynamics
Following initial administration, ZOLADEX causes an initial increase in serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels with subsequent increases in serum levels of testosterone. Chronic administration of ZOLADEX leads to sustained suppression of pituitary gonadotropins, and serum levels of testosterone consequently fall into the range normally seen in surgically castrated men approximately 21 days after initiation of therapy. This leads to accessory sex organ regression.
In clinical trials using ZOLADEX 3.6 mg with follow-up of more than 2 years, suppression of serum testosterone to castrate levels has been maintained for the duration of therapy.
12.3 Pharmacokinetics
Absorption
The pharmacokinetics of ZOLADEX have been determined in healthy male volunteers and patients. In healthy males, radiolabeled goserelin was administered as a single 250 mcg (aqueous solution) dose by the subcutaneous route. The absorption of radiolabeled drug was rapid, and the peak blood radioactivity levels occurred between 0.5 and 1.0 hour after dosing.
The overall pharmacokinetic profile of goserelin following administration of a ZOLADEX 10.8 mg depot to patients with prostate cancer was determined. The initial release of goserelin from the depot was relatively rapid resulting in a peak concentration at 2 hours after dosing. From Day 4 until the end of the 12-week dosing interval, the sustained release of goserelin from the depot produced reasonably stable systemic exposure. Mean (Standard Deviation) pharmacokinetic data are presented in Table 4. There is no clinically significant accumulation of goserelin following administration of four depots administered at 12-week intervals.
Table 4 GOSERELIN PHARMACOKINETIC PARAMETERS FOR THE 10.8 MG DEPOT SD = standard deviation
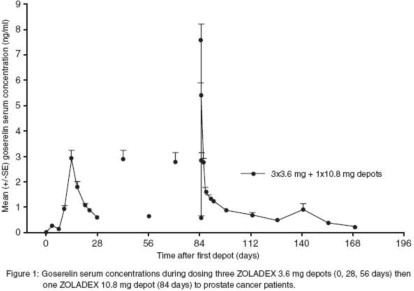
Parameter N Mean (SD) Systemic clearance (mL/min) 41 121 (42.4) Cmax (ng/mL) 41 8.85 (2.83) Tmax (h) 41 1.80 (0.34) Cmin (ng/mL) 44 0.37 (0.21) Serum goserelin concentrations in prostate cancer patients administered three 3.6 mg depots followed by one 10.8 mg depot are displayed in Figure 1. The profiles for both formulations are primarily dependent upon the rate of drug release from the depots. For the 3.6 mg depot, mean concentrations gradually rise to reach a peak of about 3 ng/mL at around 15 days after administration and then decline to approximately 0.5 ng/mL by the end of the treatment period. For the 10.8 mg depot, mean concentrations increase to reach a peak of about 8 ng/mL within the first 24 hours and then decline rapidly up to Day 4. Thereafter, mean concentrations remain relatively stable in the range of about 0.3 to 1 ng/mL up to the end of the treatment period.
Administration of four ZOLADEX 10.8 mg depots to patients with prostate cancer resulted in testosterone levels that were suppressed to and maintained within the range normally observed in surgically castrated men (0 – 1.73 nmol/L or 0-50 ng/dL), over the dosing interval in approximately 91% (145/160) of patients studied. In 6 of 15 patients that escaped from castrate range, serum testosterone levels were maintained below 2.0 nmol/L (58 ng/dL) and in only one of the 15 patients did the depot completely fail to maintain serum testosterone levels to within the castrate range over a 336-day period (4 depot injections). In the 8 additional patients, a transient escape was followed 14 days later by a level within the castrate range.
Distribution
The apparent volume of distribution determined after subcutaneous administration of 250 mcg aqueous solution of goserelin was 44.1 ± 13.6 liters for healthy males. The plasma protein binding of goserelin was found to be 27%.
Metabolism
Metabolism of goserelin, by hydrolysis of the C-terminal amino acids, is the major clearance mechanism. The major circulating component in serum appeared to be 1–7 fragment, and the major component present in urine of one healthy male volunteer was 5-10 fragment. The metabolism of goserelin in humans yields a similar but narrow profile of metabolites to that found in other species. All metabolites found in humans have also been found in toxicology species.
Excretion
Clearance of goserelin following subcutaneous administration of a radiolabeled solution of goserelin was very rapid and occurred via a combination of hepatic and urinary excretion. More than 90% of a subcutaneous radiolabeled solution formulation dose of goserelin was excreted in urine. Approximately 20% of the dose recovered in urine was accounted for by unchanged goserelin.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Subcutaneous implantation of goserelin in male and female rats once every 4 weeks for 1 year and recovery for 23 weeks at doses of about 80 and 150 mcg/kg (males) and 50 and 100 mcg/kg (females) daily resulted in an increased incidence of pituitary adenomas. An increased incidence of pituitary adenomas was also observed following subcutaneous implant of goserelin in rats at similar dose levels for a period of 72 weeks in males and 101 weeks in females. The relevance of the rat pituitary adenomas to humans has not been established. Subcutaneous implants of goserelin every 3 weeks for 2 years delivered to mice at doses of up to 2400 mcg/kg/day resulted in an increased incidence of histiocytic sarcoma of the vertebral column and femur. Human dose/exposure multiples could not be calculated from available animal data.
Mutagenicity tests using bacterial and mammalian systems for point mutations and cytogenetic effects have provided no evidence for mutagenic potential.
Administration of goserelin led to changes that were consistent with gonadal suppression in both male and female rats as a result of its endocrine action. In male rats administered 500-1000 mcg/kg/day, a decrease in weight and atrophic histological changes were observed in the testes, epididymis, seminal vesicle and prostate gland with complete suppression of spermatogenesis. In female rats administered 50-1000 mcg/kg/day, suppression of ovarian function led to decreased size and weight of ovaries and secondary sex organs; follicular development was arrested at the antral stage and the corpora lutea were reduced in size and number. Except for the testes, almost complete histologic reversal of these effects in males and females was observed several weeks after dosing was stopped; however, fertility and general reproductive performance were reduced in those that became pregnant after goserelin was discontinued. Fertile matings occurred within 2 weeks after cessation of dosing, even though total recovery of reproductive function may not have occurred before mating took place; and, the ovulation rate, the corresponding implantation rate, and number of live fetuses were reduced.
Based on histological examination, drug effects on reproductive organs were reversible in male and female dogs administered 107-214 mcg/kg/day goserelin when drug treatment was stopped after continuous administration for 1 year. Human dose/exposure multiples could not be calculated from available animal data.
-
14 CLINICAL STUDIES
14.1 Stage B2-C Prostatic Carcinoma
The effects of hormonal treatment combined with radiation were studied in 466 patients (231 ZOLADEX + flutamide + radiation, 235 radiation alone) with bulky primary tumors confined to the prostate (stage B2) or extending beyond the capsule (stage C), with or without pelvic node involvement.
In this multicentered, controlled trial, administration of ZOLADEX (3.6 mg depot) and flutamide capsules (250 mg t.i.d.) prior to and during radiation was associated with a significantly lower rate of local failure compared to radiation alone (16% vs 33% at 4 years, P<0.001). The combination therapy also resulted in a trend toward reduction in the incidence of distant metastases (27% vs 36% at 4 years, P=0.058). Median disease-free survival was significantly increased in patients who received complete hormonal therapy combined with radiation as compared to those patients who received radiation alone (4.4 vs 2.6 years, P<0.001). Inclusion of normal PSA level as a criterion for disease-free survival also resulted in significantly increased median disease-free survival in patients receiving the combination therapy (2.7 vs 1.5 years, P<0.001).
14.2 Prostatic Carcinoma
In two controlled clinical trials, 160 patients with advanced prostate cancer were randomized to receive either one 3.6 mg ZOLADEX implant every four weeks or a single 10.8 mg ZOLADEX implant every 12 weeks. Mean serum testosterone suppression was similar between the two arms. PSA falls at three months were 94% in patients who received the 10.8 mg implant and 92.5% in patients that received three 3.6 mg implants.
Periodic monitoring of serum testosterone levels should be considered if the anticipated clinical or biochemical response to treatment has not been achieved. A clinical outcome similar to that produced with the use of the 3.6 mg implant administered every 28 days is predicted with ZOLADEX 10.8 mg implant administered every 12 weeks (84 days). Total testosterone was measured by the DPC Coat-A-Count radioimmunoassay method which, as defined by the manufacturers, is highly specific and accurate. Acceptable variability of approximately 20% at low testosterone levels has been demonstrated in the clinical studies performed with the ZOLADEX 10.8 mg depot.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
ZOLADEX 10.8 mg implant is supplied as a sterile and totally biodegradable D,L-lactic and glycolic acids copolymer impregnated with 11.3 mg goserelin acetate equivalent to 10.8 mg of goserelin in a disposable syringe device fitted with a 14-gauge x 36 +/- 0.5 mm siliconized hypodermic needle with protective sleeve [SafeSystem® Syringe] (NDC 70720-951-30). The unit is sterile and comes in a sealed, light- and moisture-proof, aluminum foil laminate pouch containing a desiccant capsule. Store at room temperature (do not exceed 25°C [77°F]).
-
17 PATIENT COUNSELING INFORMATION
17.1 Males
The use of ZOLADEX in patients at particular risk of developing ureteral obstruction or spinal cord compression should be considered carefully and the patients monitored closely during the first month of therapy. Patients with ureteral obstruction or spinal cord compression should have appropriate treatment prior to initiation of ZOLADEX therapy [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
The use of GnRH agonists may cause a reduction in bone mineral density. In men, data suggest the use of a bisphosphonate in combination with a GnRH agonist may reduce bone mineral loss [see Adverse Reactions (6.5)].
Patients should be informed that diabetes, or loss of glycemic control in patients with pre-existing diabetes, has been reported during treatment with GnRH agonists, including ZOLADEX. Therefore, consideration should be given to monitoring blood glucose and/or glycosylated hemoglobin (HbA1c) periodically in patients receiving ZOLADEX [see Warnings and Precautions (5.3)].
A small increased risk of developing myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH agonists in men. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice [see Warnings and Precautions (5.4)].
Injection site injury has been reported following injection of ZOLADEX. Inform patients to contact their doctor immediately if they experience any of the following symptoms: abdominal pain, abdominal distension, dyspnea, dizziness, hypotension and/or any altered levels of consciousness [see Warnings and Precautions (5.6)].
ZOLADEX® and SAFESYSTEM® are registered trademarks of AstraZeneca or its affiliates and are used herein under license.
©2020 TerSera Therapeutics LLC
Distributed by: TerSera Therapeutics LLC, Deerfield, IL 60015
TerSera®
therapeuticsRev. 12/2020
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ZOLADEX
goserelin implantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70720-951 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength goserelin acetate (UNII: 6YUU2PV0U8) (goserelin - UNII:0F65R8P09N) goserelin 10.8 mg Inactive Ingredients Ingredient Name Strength Glycolic Acid (UNII: 0WT12SX38S) Lactic Acid, DL- (UNII: 3B8D35Y7S4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70720-951-30 1 in 1 CARTON 01/26/2018 1 1 in 1 POUCH 1 1 in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020578 01/26/2018 Labeler - TerSera Therapeutics LLC (080226115)