Label: SULFAMYLON- mafenide acetate cream
- NDC Code(s): 16571-723-12, 16571-723-48, 16571-723-60
- Packager: Rising Pharma Holdings, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
SULFAMYLON Cream is a soft, white, nonstaining, water-miscible, anti-infective cream for topical administration to burn wounds.
SULFAMYLON Cream spreads easily, and can be washed off readily with water. It has a slight acetic odor. Each gram of SULFAMYLON Cream contains mafenide acetate equivalent to 85 mg of the base. The cream vehicle consists of cetyl alcohol, steryl alcohol, cetyl esters wax, polyoxyl 40 stearate, polyoxyl 8 stearate, glycerin, and water, with methylparaben, propylparaben, sodium metabisulfite, and edetate disodium as preservatives.
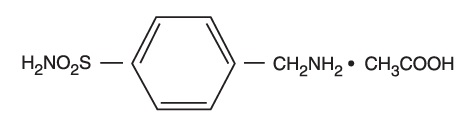
Chemically, mafenide acetate is α-Amino-ρ-toluenesulfonamide monoacetate and has the following structural formula:
-
CLINICAL PHARMACOLOGY
SULFAMYLON Cream, applied topically produces a marked reduction in the bacterial population present in the avascular tissues of second- and third-degree burns. Reduction in bacterial growth after application of SULFAMYLON Cream has also been reported to permit spontaneous healing of deep partial-thickness burns, and thus prevent conversion of burn wounds from partial-thickness to full-thickness. It should be noted, however, that delayed eschar separation has occurred in some cases.
Absorption and Metabolism
Applied topically, SULFAMYLON Cream diffuses through devascularized areas, is absorbed, and rapidly converted to a metabolite (ρ-carboxybenzenesulfonamide) which is cleared through the kidneys. SULFAMYLON is active in the presence of pus and serum, and its activity is not altered by changes in the acidity of the environment.
Antibacterial Activity
SULFAMYLON exerts bacteriostatic action against many gram-negative and gram-positive organisms, including Pseudomonas aeruginosa and certain strains of anaerobes. - INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Fatal hemolytic anemia with disseminated intravascular coagulation, presumably related to a glucose-6-phosphate dehydrogenase deficiency, has been reported following therapy with SULFAMYLON Cream.
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people. -
PRECAUTIONS
SULFAMYLON and its metabolite, ρ-carboxybenzenesulfonamide, inhibit carbonic anhydrase, which may result in metabolic acidosis, usually compensated by hyperventilation. In the presence of impaired renal function, high blood levels of SULFAMYLON and its metabolite may exaggerate the carbonic anhydrase inhibition. Therefore, close monitoring of acid-base balance is necessary, particularly in patients with extensive second-degree or partial-thickness burns and in those with pulmonary or renal dysfunction. Some burn patients treated with SULFAMYLON Cream have also been reported to manifest an unexplained syndrome of marked hyperventilation with resulting respiratory alkalosis (slightly alkaline blood pH, low arterial pCO2 , and decreased total CO2); change in arterial pO2 is variable. The etiology and significance of these findings are unknown.
Mafenide acetate cream should be used with caution in burn patients with acute renal failure.
SULFAMYLON Cream should be administered with caution to patients with history of hypersensitivity to mafenide. It is not known whether there is cross sensitivity to other sulfonamides.
Fungal colonization in and below eschar may occur concomitantly with reduction of bacterial growth in the burn wound. However, fungal dissemination through the infected burn wound is rare.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate the drug’s potential in these areas.
Pregnancy
Animal reproduction studies have not been conducted with SULFAMYLON. It is also not known whether SULFAMYLON can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Therefore, the preparation is not recommended for the treatment of a women of childbearing potential, unless the burned area covers more than 20% of the total body surface, or the need for the therapeutic benefit of SULFAMYLON Cream is, in the physician’s judgment, greater than the possible risk to the fetus.
Nursing Mothers
It is not known whether mafenide acetate is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reaction in nursing infants from SULFAMYLON, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Same as for adults. (See DOSAGE AND ADMINISTRATION.) -
ADVERSE REACTIONS
It is frequently difficult to distinguish between an adverse reaction to SULFAMYLON Cream and the effect of a severe burn. A single case of bone marrow depression and a single case of acute attack of porphyria have been reported following therapy with SULFAMYLON Cream. Fatal hemolytic anemia with disseminated intravascular coagulation, presumably related to a glucose-6-phosphate dehydrogenase deficiency, has been reported following therapy with SULFAMYLON Cream.
Dermatologic: The most frequently reported reaction was pain on application or a burning sensation. Rare occurrences are excoriation of new skin and bleeding of skin.
Allergic: Rash itching, facial edema, swelling, hive, blisters, erythema, and eosinophilia.
Respiratory: Tachypnea or hyperventilation, decrease in arterial pCO2.
Metabolic: Acidosis, increase in serum chloride.
Accidental ingestion of SULFAMYLON Cream has been reported to cause diarrhea.
To report SUSPECTED ADVERSE REACTIONS, contact Rising Pharma Holdings, Inc. at 1-844-874-7464 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. -
DOSAGE AND ADMINISTRATION
Prompt institution of appropriate measures for controlling shock and pain is of prime importance. The burn wounds are then cleansed and debrided, and SULFAMYLON Cream is applied with a sterile gloved hand. Satisfactory results can be achieved with application of the cream once or twice daily, to a thickness of approximately 1/16 inch; thicker application is not recommended. The burned areas should be covered with SULFAMYLON Cream at all times. Therefore, whenever necessary, the cream should be reapplied to any areas from which it has been removed (e.g., by patient activity). The routine of administration can be accomplished in minimal time, since dressings usually are not required, if individual patient demands make them necessary, however, only a thin layer of dressings should be used.
When feasible, the patient should be bathed daily to aid in debridement. A whirlpool bath is particularly helpful, but the patient may be bathed in bed or in a shower.
The duration of therapy with SULFAMYLON Cream depends on each patient’s requirements. Treatment is usually continued until healing is progressing well or until the burn site is ready for grafting. SULFAMYLON Cream should not be withdrawn from the therapeutic regimen while there is the possibility of infection. However, if allergic manifestations occur during treatment with SULFAMYLON Cream, discontinuation of treatment should be considered.
If acidosis occurs and becomes difficult to control, particularly in patients with pulmonary dysfunction, discontinuing therapy SULFAMYLON Cream for 24 to 48 hours while continuing fluid therapy may aid in restoring acid-base balance.
-
HOW SUPPLIED
16 oz. Plastic Jar (453.6 g) - NDC 16571-723-48
4 oz. Tube (113.4 g) - NDC 16571-723-12
2 oz. Tube (56.7 g) - NDC 16571-723-60
Avoid exposure to excessive heat (temperature above 104° F or 40° C).
For External Use Only.
Keep this and all medications out of the reach of children.
Rx only
Manufactured for:
Rising Pharma Holdings, Inc.
East Brunswick, NJ 08816
Revised: 08/2020
PIR72348-00 -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 16571-723-60 Rising®
SULFAMYLON® Cream
(mafenide acetate cream, USP)
Rx only
2 oz. (56.7 g) For Topical Use Only
Each gram contains mafenide acetate equivalent to 85 mg base in a water-miscible cream of cetyl alcohol, stearyl alcohol, cetyl esters wax, polyoxyl 40 stearate, polyoxyl 8 stearate, glycerin, and water, with methylparaben, propylparaben, sodium metabisulfite, and edetate disodium as preservatives.
For dosage and administration, read accompanying package insert.
Avoid exposure to excessive heat (temperatures above 104°F or 40°C).
For external use only.
Keep this and all medications out of the reach of children.

-
INGREDIENTS AND APPEARANCE
SULFAMYLON
mafenide acetate creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16571-723 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAFENIDE ACETATE (UNII: RQ6LP6Z0WY) (MAFENIDE - UNII:58447S8P4L) MAFENIDE 85 mg in 1 g Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) POLYOXYL 40 STEARATE (UNII: 13A4J4NH9I) PEG-8 STEARATE (UNII: 2P9L47VI5E) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM METABISULFITE (UNII: 4VON5FNS3C) EDETATE DISODIUM (UNII: 7FLD91C86K) Product Characteristics Color WHITE (soft white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16571-723-48 453.6 g in 1 JAR; Type 0: Not a Combination Product 05/18/2020 2 NDC:16571-723-12 1 in 1 CARTON 05/18/2020 2 113.4 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:16571-723-60 1 in 1 CARTON 05/18/2020 3 56.7 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA016763 05/18/2020 Labeler - Rising Pharma Holdings, Inc. (116880195) Establishment Name Address ID/FEI Business Operations Kavis Pharma LLC 119014974 ANALYSIS(16571-723) , LABEL(16571-723) , MANUFACTURE(16571-723) , PACK(16571-723)

