Label: CURAE- levonorgestrel tablet
- NDC Code(s): 73358-241-01
- Packager: Curae Pharma360 Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
Sexually transmitted diseases (STDs) alert
This product does not protect against HIV/AIDS or other STDs
Ask a doctor or pharmacist before use if you are taking efavirenz (HIV medication) or rifampin (tuberculosis treatment) or medication for seizures (epilepsy). These medications may reduce the effectiveness of levonorgestrel.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
-
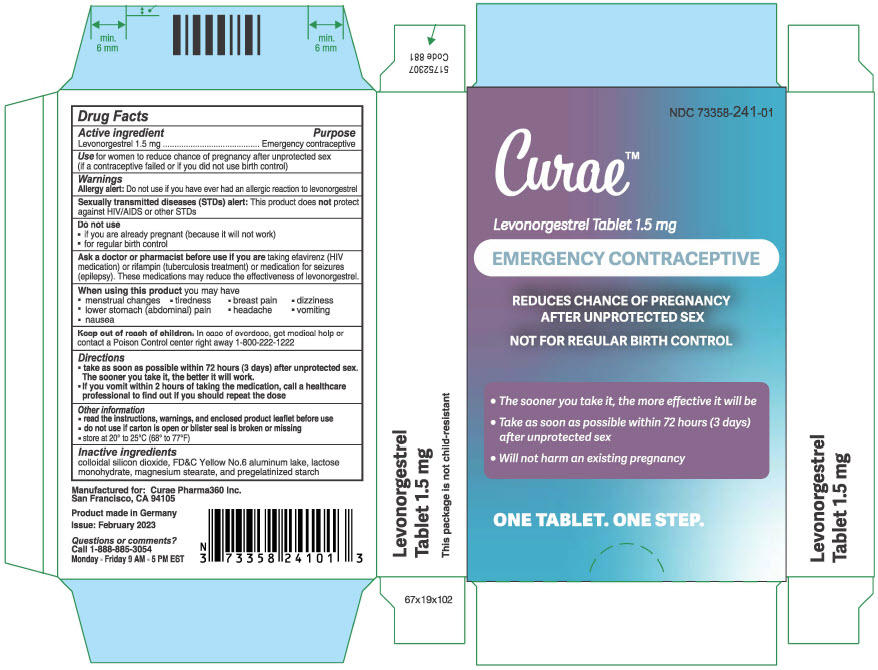
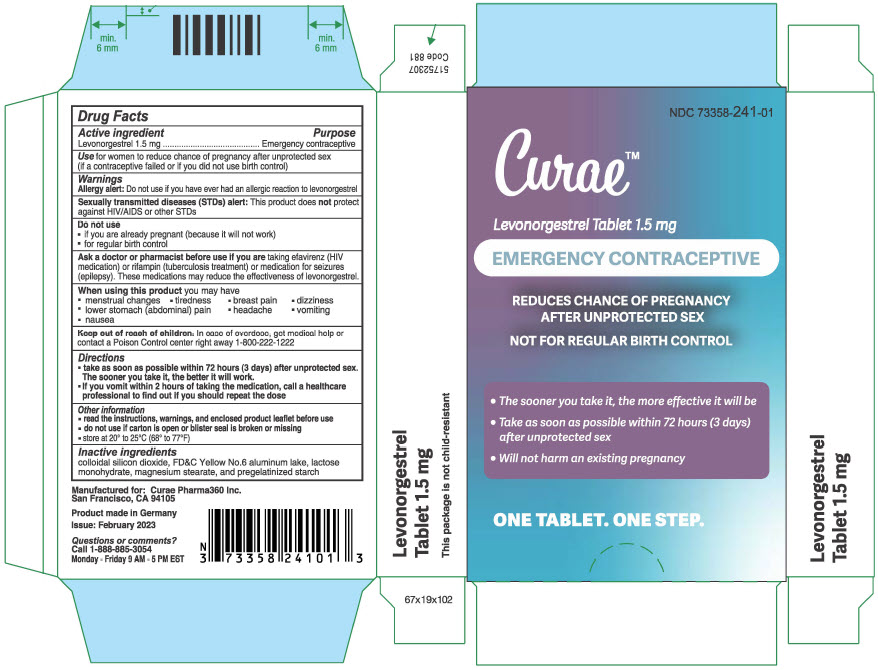
PRINCIPAL DISPLAY PANEL - 1.5 mg Tablet Blister Pack Carton
NDC 73358-241-01
Curae™
Levonorgestrel Tablet 1.5 mg
EMERGENCY CONTRACEPTIVE
REDUCES CHANCE OF PREGNANCY
AFTER UNPROTECTED SEXNOT FOR REGULAR BIRTH CONTROL
- The sooner you take it, the more effective it will be
-
Take as soon as possible within 72 hours (3 days)
after unprotected sex - Will not harm an existing pregnancy
ONE TABLET. ONE STEP.
-
INGREDIENTS AND APPEARANCE
CURAE
levonorgestrel tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73358-241 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levonorgestrel (UNII: 5W7SIA7YZW) (Levonorgestrel - UNII:5W7SIA7YZW) Levonorgestrel 1.5 mg Inactive Ingredients Ingredient Name Strength Lactose Monohydrate (UNII: EWQ57Q8I5X) Starch, Corn (UNII: O8232NY3SJ) Magnesium Stearate (UNII: 70097M6I30) Silicon Dioxide (UNII: ETJ7Z6XBU4) Fd&C Yellow No. 6 (UNII: H77VEI93A8) Product Characteristics Color ORANGE (Light peach) Score no score Shape ROUND (convex) Size 8mm Flavor Imprint Code 17 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73358-241-01 1 in 1 CARTON 03/01/2023 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202380 03/01/2023 Labeler - Curae Pharma360 Inc. (080719852) Registrant - Naari Pte. Limited (659345996)