Label: TRUDHESA- dihydroergotamine mesylate spray, metered
- NDC Code(s): 77530-725-00, 77530-725-04
- Packager: Impel Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TRUDHESA safely and effectively. See full prescribing information for TRUDHESA.
TRUDHESA® (dihydroergotamine mesylate) nasal spray
Initial U.S. Approval: 1946WARNING: PERIPHERAL ISCHEMIA FOLLOWING COADMINISTRATION WITH POTENT CYP3A4 INHIBITORS
See full prescribing information for complete boxed warning.
Serious and/or life-threatening peripheral ischemia has been associated with the coadministration of dihydroergotamine with strong CYP3A4 inhibitors. Because CYP3A4 inhibition elevates the serum levels of dihydroergotamine, the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of TRUDHESA with strong CYP3A4 inhibitors is contraindicated. (4, 5.1, 7.1)
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- The recommended dose of TRUDHESA is 1.45 mg (administered as one metered spray of 0.725 mg into each nostril). (2.1)
- The dose may be repeated, if needed, a minimum of 1 hour after the first dose. Do not use more than 2 doses within a 24-hour period or 3 doses within 7 days. (2.1)
- Prior to initiation, a cardiovascular evaluation is recommended. (2.2)
- TRUDHESA is for nasal administration only. (2.3)
- Assemble and prime (i.e., pumped 4 times) before use. (2.3)
- Use TRUDHESA immediately after priming and then discard. (2.3)
DOSAGE FORMS AND STRENGTHS
Nasal spray: 0.725 mg dihydroergotamine mesylate per spray. (3)
CONTRAINDICATIONS
- Concomitant use of strong CYP3A4 inhibitors (4)
- Patients with ischemic heart disease or coronary artery vasospasm (4)
- Patients with uncontrolled hypertension, peripheral arterial diseases, sepsis, following vascular surgery, or severe hepatic or renal impairment (4)
- Patients with hypersensitivity to ergot alkaloids (4)
- Concomitant use of other 5-HT1 agonists (e.g., sumatriptan) or ergotamine-containing or ergot-type medications within 24 hours (4)
- Concomitant use of peripheral and central vasoconstrictors (4)
WARNINGS AND PRECAUTIONS
- Myocardial Ischemia and/or Infarction, Other Cardiac Adverse Reactions, and Fatalities: In patients with risk factors, consider 1st dose administration under medical supervision with electrocardiogram. (2.2, 5.2)
- Cerebrovascular Adverse Reactions and Fatalities: Cerebral hemorrhage, subarachnoid hemorrhage and stroke have been reported; discontinue TRUDHESA if suspected. (5.3)
- Other Vasospasm Related Adverse Reactions: TRUDHESA may cause vasospasm or elevation in blood pressure. Discontinue if signs or symptoms of vasoconstriction develop. (5.4, 5.5)
- Medication Overuse Headache: Detoxification may be necessary. (5.6)
- Preterm Labor: Advise pregnant women of the risk. (5.7, 8.1)
- Fibrotic Complications: Pleural and retroperitoneal fibrosis have been reported following prolonged daily use of dihydroergotamine mesylate. Administration of TRUDHESA should not exceed the dosing guidelines or be used for chronic daily administration. (5.8)
- Local Irritation: If severe local irritation occurs for no other attributable reasons, suspend TRUDHESA until resolution. (5.9)
ADVERSE REACTIONS
Most common adverse reactions (greater than 1%) were rhinitis, nausea, altered sense of taste, application site reactions, dizziness, vomiting, somnolence, pharyngitis, and diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Impel Pharmaceuticals Inc. at 1-833-878-3437 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: PERIPHERAL ISCHEMIA FOLLOWING COADMINISTRATION WITH STRONG CYP3A4 INHIBITORS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
2.2 Assessment Prior to First Dose
2.3 Important Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Peripheral Ischemia Following Coadministration with Strong CYP3A4 Inhibitors
5.2 Myocardial Ischemia and/or Infarction, Other Cardiac Adverse Reactions, and Fatalities
5.3 Cerebrovascular Adverse Reactions and Fatalities
5.4 Other Vasospasm Related Adverse Reactions
5.5 Increase in Blood Pressure
5.6 Medication Overuse Headache
5.7 Preterm Labor
5.8 Fibrotic Complications
5.9 Local Irritation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 CYP3A4 Inhibitors
7.2 Triptans
7.3 Beta Blockers
7.4 Vasoconstrictors
7.5 Nicotine
7.6 Selective Serotonin Reuptake Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
10.1 Symptoms
10.2 Treatment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: PERIPHERAL ISCHEMIA FOLLOWING COADMINISTRATION WITH STRONG CYP3A4 INHIBITORS
Serious and/or life-threatening peripheral ischemia has been associated with the coadministration of dihydroergotamine with strong CYP3A4 inhibitors. Because CYP3A4 inhibition elevates the serum levels of dihydroergotamine, the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of TRUDHESA with strong CYP3A4 inhibitors is contraindicated [see Contraindications (4), Warnings and Precautions (5.1), and Drug Interactions (7.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
The recommended dose of TRUDHESA is 1.45 mg administered as two metered sprays into the nose (one spray of 0.725 mg into each nostril).
The dose may be repeated, if needed, a minimum of 1 hour after the first dose. Do not use more than 2 doses of TRUDHESA within a 24-hour period or 3 doses within a 7-day period.
2.2 Assessment Prior to First Dose
Prior to initiation of TRUDHESA, a cardiovascular evaluation is recommended [see Warnings and Precautions (5.2)]. For patients with risk factors predictive of coronary artery disease who are determined to have a satisfactory cardiovascular evaluation, it is strongly recommended that administration of the first dose of TRUDHESA take place in the setting of an equipped healthcare facility.
2.3 Important Administration Instructions
TRUDHESA is for nasal administration only and must not be injected.
TRUDHESA must be assembled prior to use (see Instructions for Use). Use or discard TRUDHESA within 8 hours once the vial has been opened or the product has been assembled.
Prime the assembled TRUDHESA before initial use by releasing 4 sprays. Use TRUDHESA immediately after priming. Discard TRUDHESA immediately after use. Open and prepare a new TRUDHESA if an additional dose is needed.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
TRUDHESA is contraindicated in patients:
- with concomitant use of strong CYP3A4 inhibitors, such as protease inhibitors (e.g., ritonavir, nelfinavir, or indinavir), macrolide antibiotics (e.g., erythromycin or clarithromycin), and antifungals (ketoconazole or itraconazole) [see Warnings and Precautions (5.1) and Drug Interactions (7.1)]
- with ischemic heart disease (angina pectoris, history of myocardial infarction, or documented silent ischemia) or patients who have clinical symptoms or findings consistent with coronary artery vasospasm, including Prinzmetal's variant angina [see Warnings and Precautions (5.4)]
- with uncontrolled hypertension [see Warnings and Precautions (5.5)]
- with peripheral arterial disease
- with sepsis
- following vascular surgery
- with severe hepatic impairment
- with severe renal impairment
- with known hypersensitivity to ergot alkaloids
- with recent use (i.e., within 24 hours) of other 5-HT1 agonists (e.g., sumatriptan) or ergotamine-containing or ergot-type medications [see Drug Interactions (7.2)]
- with concomitant use of peripheral and central vasoconstrictors because the combination may result in additive or synergistic elevation of blood pressure [see Warnings and Precautions (5.5)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Peripheral Ischemia Following Coadministration with Strong CYP3A4 Inhibitors
Serious and/or life-threatening peripheral ischemia has been associated with the coadministration of dihydroergotamine with strong CYP3A4 inhibitors, including protease inhibitors, macrolide antibiotics, and antifungals. Because CYP3A4 inhibition elevates the serum levels of dihydroergotamine, the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of TRUDHESA with strong CYP3A4 inhibitors is contraindicated [see Contraindications (4) and Drug Interactions (7.1)].
5.2 Myocardial Ischemia and/or Infarction, Other Cardiac Adverse Reactions, and Fatalities
The potential for adverse cardiac adverse reactions exists with TRUDHESA treatment. Serious adverse cardiac events, including some that have been fatal, have occurred following use of dihydroergotamine mesylate. These events have included acute myocardial infarction, life-threatening disturbances of cardiac rhythm (e.g., ventricular tachycardia and ventricular fibrillation), coronary artery vasospasm, and transient myocardial ischemia.
Prior to initiation of TRUDHESA, a cardiovascular evaluation is recommended to determine if the patient is free of coronary artery and ischemic myocardial disease or other significant underlying cardiovascular disease. If, during the cardiovascular evaluation, the patient's medical history (including risk factors), or electrocardiographic investigation, findings are consistent with coronary artery vasospasm or myocardial ischemia, TRUDHESA should not be administered [see Contraindications (4)].
For patients with risk factors predictive of coronary artery disease (e.g., hypertension, hypercholesterolemia, smoker, obesity, diabetes, strong family history of coronary artery disease, females who are surgically or physiologically postmenopausal, or males who are over 40 years of age) who are determined to have a satisfactory cardiovascular evaluation, it is strongly recommended that administration of the first dose of TRUDHESA take place in the setting of an equipped healthcare facility, unless the patient has previously received dihydroergotamine mesylate. During the interval immediately following the first use of TRUDHESA, an electrocardiogram is recommended in those patients with risk factors because ischemia can occur in the absence of clinical symptoms.
5.3 Cerebrovascular Adverse Reactions and Fatalities
The potential for adverse cerebrovascular adverse reactions exists with TRUDHESA treatment. Cerebral hemorrhage, subarachnoid hemorrhage, stroke, and other cerebrovascular events have been reported in patients treated with dihydroergotamine mesylate; and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the dihydroergotamine mesylate having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine, when they were not. It should be noted that patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, hemorrhage, transient ischemic attack). Discontinue TRUDHESA if a cerebrovascular event is suspected.
5.4 Other Vasospasm Related Adverse Reactions
TRUDHESA, like other ergot alkaloids, may cause vasospastic reactions other than coronary artery vasospasm. Myocardial, peripheral vascular, and colonic ischemia have been reported with dihydroergotamine mesylate.
Dihydroergotamine mesylate associated vasospastic phenomena may also cause muscle pains, numbness, coldness, pallor, and cyanosis of the digits. In patients with compromised circulation, persistent vasospasm may result in gangrene or death. TRUDHESA should be discontinued immediately if signs or symptoms of vasoconstriction develop.
Patients who experience other symptoms or signs suggestive of decreased arterial flow, such as ischemic bowel syndrome or Raynaud's syndrome, following the use of any 5-HT agonist, including TRUDHESA, should be evaluated by a healthcare provider.
5.5 Increase in Blood Pressure
Significant elevation in blood pressure has been reported on rare occasions in patients with and without a history of hypertension treated with dihydroergotamine mesylate. TRUDHESA is contraindicated in patients with uncontrolled hypertension [see Contraindications (4)].
An 18% increase in mean pulmonary artery pressure was seen following dosing with another 5-HT1 agonist in a study evaluating subjects undergoing cardiac catheterization.
5.6 Medication Overuse Headache
Overuse of acute migraine drugs (e.g., ergotamines, triptans, opioids, or a combination of these drugs for 10 or more days per month) may lead to exacerbation of headache (i.e., medication overuse headache). Medication overuse headache may present as migraine-like daily headaches or as a marked increase in frequency of migraine attacks. Detoxification of patients including withdrawal of the overused drugs and treatment of withdrawal symptoms (which often includes a transient worsening of headache) may be necessary.
5.7 Preterm Labor
Based on the mechanism of action of dihydroergotamine and findings from the published literature, TRUDHESA may cause preterm labor. Avoid use of TRUDHESA during pregnancy [see Use in Specific Populations (8.1)].
5.8 Fibrotic Complications
The potential for fibrotic complications exists with TRUDHESA treatment. There have been reports of pleural and retroperitoneal fibrosis in patients following prolonged daily use of dihydroergotamine mesylate. Rarely, prolonged daily use of other ergot alkaloid drugs has been associated with cardiac valvular fibrosis. Rare cases have also been reported in association with the use of dihydroergotamine mesylate; however, in those cases, patients also received drugs known to be associated with cardiac valvular fibrosis.
Administration of TRUDHESA should not exceed the dosing guidelines and should not be used for chronic daily administration [see Dosage and Administration (2.1)].
5.9 Local Irritation
Local irritative symptoms were reported in 52% of patients treated with at least one dose of TRUDHESA in an open-labeled trial, which allowed repeated use of TRUDHESA over 6 to 12 months. The most common local irritative symptoms (at least 1% of patients) were nasopharyngitis (21%), rhinitis (19%), nasal discomfort (7%), product taste abnormal/dysgeusia (6%), sinusitis (5%), sinus discomfort (4%), olfactory test abnormal [defined based on a change in score at a prespecified threshold on the University of Pennsylvania Smell Identification Test (UPSIT)] (4%), epistaxis (3%), pharyngitis (3%), nasal mucosal disorder (2%), change in smell (1%), ear discomfort (1%), and rhinorrhea (1%). If a severe local irritation event occurs for no other attributable reasons, TRUDHESA should be temporarily suspended until the event resolves. If the event does not resolve, or it recurs with re-challenge, TRUDHESA should be discontinued permanently. Administration of TRUDHESA should not exceed the dosing guidelines and should not be used for chronic daily administration [see Dosage and Administration (2.1)].
Nasal tissue in animals treated with dihydroergotamine mesylate daily showed mild mucosal irritation characterized by mucous cell and transitional cell hyperplasia and squamous cell metaplasia. Changes in rat nasal mucosa at 64 weeks were less severe than at 13 weeks. Local effects on respiratory tissue after chronic intranasal dosing in animals have not been evaluated.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Peripheral Ischemia Following Coadministration with Strong CYP3A4 Inhibitors [see Boxed Warning and Warnings and Precautions (5.1)]
- Myocardial Ischemia and/or Infarction, Other Adverse Cardiac Events, and Fatalities [see Warnings and Precautions (5.2)]
- Cerebrovascular Adverse Reactions and Fatalities [see Warnings and Precautions (5.3)]
- Other Vasospasm Related Adverse Reactions [see Warnings and Precautions (5.4)]
- Increase in Blood Pressure [see Warnings and Precautions (5.5)]
- Medication Overuse Headache [see Warnings and Precautions (5.6)]
- Preterm Labor [see Warnings and Precautions (5.7)]
- Fibrotic Complications [see Warnings and Precautions (5.8)]
- Local Irritation [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Placebo-Controlled Trials with Dihydroergotamine (DHE) Mesylate Nasal Spray [see Clinical Studies (14)]
Of the 1,796 patients and subjects treated with DHE nasal spray doses 2 mg or less in U.S. and foreign clinical studies, 26 (1.4%) discontinued because of adverse events. The adverse events associated with discontinuation were, in decreasing order of frequency: rhinitis (13), dizziness (2), facial edema (2), and one patient each due to cold sweats, accidental trauma, depression, elective surgery, somnolence, allergy, vomiting, hypotension, and paraesthesia.
Table 1 summarizes the incidence rates of adverse reactions reported by at least 1% of patients who received DHE nasal spray for the treatment of migraine during placebo-controlled, double-blind clinical studies and were more frequent than in those patients receiving placebo. The most commonly reported adverse reactions (greater than 1% of patients who received DHE nasal spray) were rhinitis, nausea, altered sense of taste, application site reactions, dizziness, vomiting, somnolence, pharyngitis, and diarrhea. In most instances these events were transient and self-limited and did not result in patient discontinuation from a study.
Table 1 Adverse Reactions Reported by at Least 1% of the DHE Nasal Spray Treated Patients and Occurred more Frequently than in the Placebo-Group in the Migraine Placebo-Controlled Trials DHE Nasal Spray
N=597
%Placebo
N=631
%Respiratory System Rhinitis 26 7 Pharyngitis 3 1 Gastrointestinal System Nausea 10 4 Vomiting 4 1 Diarrhea 2 <1 Special Senses, Other Altered Sense of Taste 8 1 Application Site Application Site Reaction 6 2 Central and Peripheral Nervous System Dizziness 4 2 Somnolence 3 2 Body as a Whole, General Hot Flashes 1 <1 Asthenia 1 0 Musculoskeletal System Stiffness 1 <1 Adverse Reactions in Studies with TRUDHESA
An open-label study in adults (18 to 66 years of age) was conducted to evaluate the safety and tolerability of TRUDHESA, repeated use of TRUDHESA was allowed over the course of 6 to 12 months. A total of 354 patients with migraine received at least one dose of TRUDHESA. One hundred and eighty-five patients treated on average at least two migraines per month for 6 months, and 55 patients treated on average at least two migraines per month for 12 months. Of the patients who received at least one dose of TRUDHESA, 185 (52.3%) patients experienced local irritative symptoms. Of these, the most common local irritative symptoms were nasopharyngitis, rhinitis, nasal discomfort, product taste abnormal/dysgeusia, sinusitis, sinus discomfort, olfactory test abnormal, epistaxis, pharyngitis, nasal mucosal disorder, change in smell, ear discomfort, and rhinorrhea [see Warnings and Precautions (5.9)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of dihydroergotamine mesylate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Vasospasm, paresthesia, hypertension, dizziness, anxiety, dyspnea, headache, flushing, diarrhea, rash, increased sweating, and pleural and retroperitoneal fibrosis after long-term use of dihydroergotamine. Cases of myocardial infarction and stroke have been reported following the use of dihydroergotamine mesylate [see Warnings and Precautions (5.2)].
-
7 DRUG INTERACTIONS
7.1 CYP3A4 Inhibitors
There have been rare reports of serious adverse events in connection with the coadministration of intravenous administration of dihydroergotamine and strong CYP3A4 inhibitors, such as protease inhibitors (e.g., ritonavir, nelfinavir, indinavir), macrolide antibiotics (e.g., erythromycin, clarithromycin), and antifungals (e.g., ketoconazole, itraconazole), resulting in vasospasm that led to cerebral ischemia and/or ischemia of the extremities [see Warnings and Precautions (5.1)]. The use of strong CYP3A4 inhibitors with dihydroergotamine is contraindicated [see Contraindications (4)]. Administer moderate CYP3A4 inhibitors (e.g., saquinavir, nefazodone, fluconazole, grapefruit juice, fluoxetine, fluvoxamine, zileuton, clotrimazole) with caution.
7.2 Triptans
Triptans (serotonin [5-HT] 1B/1D receptor agonists) have been reported to cause coronary artery vasospasm, and its effect could be additive with TRUDHESA. Therefore, triptans and TRUDHESA should not be taken within 24 hours of each other [see Contraindications (4)].
7.3 Beta Blockers
There have been reports that propranolol may potentiate the vasoconstrictive action of ergotamine by blocking the vasodilating property of epinephrine.
7.4 Vasoconstrictors
TRUDHESA is contraindicated for use with peripheral and central vasoconstrictors because the combination may cause synergistic elevation of blood pressure [see Warnings and Precautions (5.5)].
7.5 Nicotine
Nicotine may provoke vasoconstriction in some patients, predisposing to a greater ischemic response to ergot therapy [see Warnings and Precautions (5.1, 5.5)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from published literature indicate an increased risk of preterm delivery with TRUDHESA use during pregnancy. Avoid use of TRUDHESA during pregnancy [see Warnings and Precautions (5.7)]. Data collected over decades have shown no increased risk of major birth defects or miscarriage with use of dihydroergotamine mesylate during pregnancy.
In animal studies, adverse effects on embryofetal development were observed following administration of dihydroergotamine mesylate during pregnancy (decreased fetal body weight and/or skeletal ossification) in rats and rabbits or during pregnancy and lactation in rats (decreased body weight and impaired reproductive function in the offspring) in rats at doses less than those used clinically and which were not associated with maternal toxicity (see Data).
The estimated rate of major birth defects (2.2% to 2.9%) and miscarriage (17%) among deliveries to women with migraine are similar to rates reported in women without migraine. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Intranasal administration of dihydroergotamine mesylate to pregnant rats throughout the period of organogenesis resulted in decreased fetal body weight and/or skeletal ossification at doses of 0.16 mg/day (associated with plasma exposures [AUC] less than that in humans at the maximum recommended human dose [MRHD] of 2.9 mg) or greater. A no-effect level for embryofetal toxicity was not identified in rats. Intranasal administration of dihydroergotamine mesylate to pregnant rabbits throughout organogenesis resulted in decreased skeletal ossification at 3.6 mg/day. At the no-effect dose (1.2 mg/day) for adverse effects on embryofetal development in rabbits, plasma exposures (AUC) were less than that in humans at the MRHD.
Intranasal administration of dihydroergotamine mesylate to female rats throughout pregnancy and lactation resulted in decreased body weight and impaired reproductive function (decreased mating indices) were observed in the offspring at doses of 0.16 mg/day or greater. A no-effect dose for adverse developmental effects in rats was not established.
Effects on development occurred at doses below those that produced evidence of significant maternal toxicity in these studies.
Dihydroergotamine-induced intrauterine growth retardation has been attributed to reduced uteroplacental blood flow resulting from prolonged vasoconstriction of the uterine vessels and/or increased myometrial tone.
8.2 Lactation
Risk Summary
There are no data on the presence of dihydroergotamine in human milk; however, ergotamine, a related drug, is present in human milk. There are reports of diarrhea, vomiting, weak pulse, and unstable blood pressure in breastfed infants exposed to ergotamine. TRUDHESA may reduce milk supply because it may decrease prolactin levels. Because of the potential for reduced milk supply and serious adverse events in the breastfed infant, including diarrhea, vomiting, weak pulse, and unstable blood pressure, advise patients not to breastfeed during treatment with TRUDHESA and for 3 days after the last dose. Breast milk supply during this time should be pumped and discarded.
8.5 Geriatric Use
Clinical studies of TRUDHESA and other dihydroergotamine mesylate products did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
TRUDHESA contains dihydroergotamine (as the mesylate salt), which is not a controlled substance.
9.2 Abuse
Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Currently available data have not demonstrated drug abuse with dihydroergotamine. However, cases of drug abuse in patients on other forms of ergot therapy have been reported.
9.3 Dependence
Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Currently available data have not demonstrated physical or psychological dependence with dihydroergotamine. However, cases of psychological dependence in patients on other forms of ergot therapy have been reported.
-
10 OVERDOSAGE
10.1 Symptoms
Excessive doses of dihydroergotamine may result in peripheral signs and symptoms of ergotism. In general, the symptoms of an acute TRUDHESA overdose are similar to those of an ergotamine overdose, although there may be less pronounced nausea and vomiting with TRUDHESA. The symptoms of an ergotamine overdose include the following: numbness, tingling, pain, and cyanosis of the extremities associated with diminished or absent peripheral pulses; respiratory depression; an increase and/or decrease in blood pressure, usually in that order; confusion, delirium, convulsions, and coma; and/or some degree of nausea, vomiting, and abdominal pain.
In laboratory animals, dihydroergotamine was lethal when given at intravenous doses of 44 mg/kg in mice, 130 mg/kg in rats, and 37 mg/kg in rabbits.
10.2 Treatment
Treatment includes discontinuance of the drug, local application of warmth to the affected area, the administration of vasodilators, and nursing care to prevent tissue damage. Up-to-date information about the treatment of overdosage can often be obtained from a certified Regional Poison Control Center.
-
11 DESCRIPTION
TRUDHESA (dihydroergotamine mesylate) nasal spray is a single-dose, drug-device combination product containing a dihydroergotamine mesylate drug constituent and a nasal spray device constituent.
The chemical name for dihydroergotamine mesylate is ergotaman-3', 6', 18-trione, 9,10-dihydro-12'-hydroxy-2'-methyl-5'- (phenylmethyl)-, (5'α)-, monomethanesulfonate. Its molecular weight is 679.78, and its molecular formula is C33H37N5O5∙CH4O3S.
The chemical structure is:
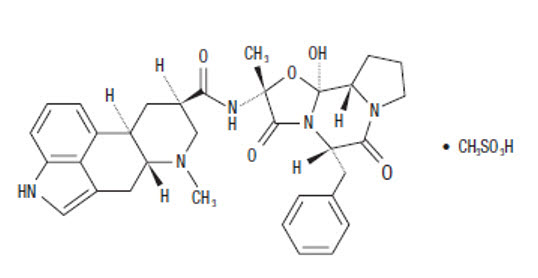
Dihydroergotamine mesylate
The drug constituent is a dihydroergotamine mesylate solution. Each milliliter (mL) of solution contains dihydroergotamine mesylate 4.0 mg (equivalent to 3.43 mg dihydroergotamine), and the following inactive ingredients: caffeine (10.0 mg), carbon dioxide (q.s.), dextrose (50.0 mg), and water (q.s. to 1.0 mL).
TRUDHESA nasal spray, after assembly and priming, delivers 0.725 mg dihydroergotamine mesylate per spray. A total dose of 1.45 mg of dihydroergotamine mesylate is delivered in 2 sprays. The nasal spray device contains hydrofluoroalkane-134a (HFA) propellant.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dihydroergotamine binds with high affinity to 5-HT1Dα and 5-HT1Dβ receptors. The therapeutic activity of dihydroergotamine in migraine is generally attributed to the agonist effects at 5-HT1D receptors.
12.2 Pharmacodynamics
Significant elevation in blood pressure has been reported in patients with and without a history of hypertension [see Warnings and Precautions (5.5)].
Dihydroergotamine possesses oxytocic properties [see Warnings and Precautions (5.7)].
12.3 Pharmacokinetics
Absorption
The mean time from dosing to maximum plasma concentration following TRUDHESA administration was approximately 0.5 hours.
Distribution
Dihydroergotamine mesylate is 93% plasma protein bound. The apparent steady-state volume of distribution is approximately 800 liters.
Elimination
Metabolism
Four dihydroergotamine mesylate metabolites have been identified in human plasma following oral administration. The major metabolite, 8'-β-hydroxy dihydroergotamine, exhibits affinity equivalent to its parent for adrenergic and 5-HT receptors and demonstrates equivalent potency in several venoconstrictor activity models, in vivo and in vitro. The other metabolites, i.e., dihydrolysergic acid, dihydrolysergic amide, and a metabolite formed by oxidative opening of the proline ring, are of minor importance. Following nasal administration, total metabolites represent only 20% to 30% of plasma AUC. The systemic clearance of dihydroergotamine mesylate following intravenous and intramuscular administration is 1.5 L/min. Quantitative pharmacokinetic characterization of the four metabolites has not been performed.
Excretion
The major excretory route of dihydroergotamine is via the bile in the feces. The total body clearance is 1.5 L/min, which reflects mainly hepatic clearance. Only 6% to 7% of unchanged dihydroergotamine is excreted in the urine after intramuscular injection. The renal clearance (0.1 L/min) is unaffected by the route of dihydroergotamine administration.
The mean apparent half-life of TRUDHESA nasal administration in healthy subjects is approximately 12 hours.
Specific Populations
No studies have been conducted on the effect of renal or hepatic impairment, gender, race, ethnicity, or pregnancy on dihydroergotamine pharmacokinetics [see Contraindications (4), Use in Specific Populations (8.1)].
Drug Interaction Studies
CYP3A4 Inhibitors
Rare reports of ergotism have been obtained from patients treated with dihydroergotamine and macrolide antibiotics (e.g., clarithromycin, erythromycin) and from patients treated with dihydroergotamine and protease inhibitors (e.g., ritonavir), presumably due to inhibition of CYP3A metabolism of ergotamine [see Contraindications (4)].
Other Drugs
The pharmacokinetics of dihydroergotamine did not appear to be significantly affected by the concomitant use of a local vasoconstrictor.
Multiple oral doses of the β-adrenoceptor antagonist propranolol, used for migraine prophylaxis, had no significant influence on the Cmax, tmax, or AUC of dihydroergotamine doses up to 4 mg. However, propranolol may potentiate the vasoconstrictive action of ergotamine [see Drug Interactions (7.3)].
The effect of oral contraceptives on the pharmacokinetics of TRUDHESA has not been studied.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Assessment of the carcinogenic potential of dihydroergotamine mesylate in mice and rats has not been assessed.
Mutagenesis
Dihydroergotamine mesylate was negative in an in vitro mutagenicity (Ames) assay and positive in in vitro chromosomal aberration (V79 Chinese hamster cell assay with metabolic activation, and human peripheral blood lymphocyte) assays. Dihydroergotamine was negative in in vivo micronucleus assays in mouse and hamster.
-
14 CLINICAL STUDIES
The efficacy of TRUDHESA is based on the relative bioavailability of TRUDHESA nasal spray compared to dihydroergotamine mesylate nasal spray in healthy subjects.
The clinical studies described below were conducted using dihydroergotamine mesylate nasal spray.
The efficacy of dihydroergotamine mesylate nasal spray for the acute treatment of migraine headaches was evaluated in four randomized, double-blind, placebo controlled studies in the U.S. The patient population for the trials was predominantly female (87%) and Caucasian (95%) with a mean age of 39 years (range 18 to 65 years). Patients treated a single moderate to severe migraine headache with a single dose of study medication and assessed pain severity over the 24 hours following treatment. Headache response was determined 0.5, 1, 2, 3 and 4 hours after dosing and was defined as a reduction in headache severity to mild or no pain. In studies 1 and 2, a four-point pain intensity scale was utilized; in studies 3 and 4, a five-point scale was used to record pain response. Although rescue medication was allowed in all four studies, patients were instructed not to use them during the four hour observation period. In studies 3 and 4, a total dose of 2 mg was compared to placebo. In studies 1 and 2, doses of 2 and 3 mg were evaluated, and showed no advantage of the higher dose for a single treatment. In all studies, patients received a regimen consisting of 0.5 mg in each nostril, repeated in 15 minutes (and again in another 15 minutes for the 3 mg dose in studies 1 and 2).
The percentage of patients achieving headache response 4 hours after treatment was significantly greater in patients receiving 2 mg doses of dihydroergotamine mesylate nasal spray compared to those receiving placebo in 3 of the 4 studies (see Table 2 and Table 3 and Figure 1 and Figure 2).
Table 2 Studies 1 and 2: Percentage of Patients with Headache Response* 2 and 4 Hours Following a Single Treatment of Study Medication (Dihydroergotamine Mesylate Nasal Spray or Placebo) N 2 hours 4 hours Study 1 Dihydroergotamine mesylate nasal spray 105 61%† 70%† Placebo 98 23% 28% Difference from Placebo 37% 42% Study 2 Dihydroergotamine mesylate nasal spray 103 47% 56%‡ Placebo 102 33% 35% Difference from Placebo 14% 21% Table 3 Studies 3 and 4: Percentage of Patients with Headache Response* 2 and 4 Hours Following a Single Treatment of Study Medication (Dihydroergotamine Mesylate Nasal Spray or Placebo) N 2 hours 4 hours Study 3 Dihydroergotamine mesylate nasal spray 50 32% 48%† Placebo 50 20% 22% Difference from Placebo 12% 26% Study 4 Dihydroergotamine mesylate nasal spray 47 30% 47% Placebo 50 20% 30% Difference from Placebo 10% 17% The Kaplan-Meier plots below (Figure 1 and Figure 2) provides an estimate of the probability that a patient will have responded to a single 2 mg dose of dihydroergotamine mesylate nasal spray as a function of the time elapsed since initiation of treatment.
- *
- The figure shows the probability over time of obtaining a response following treatment with dihydroergotamine mesylate nasal spray. Headache response was based on pain intensity as interpreted by the patient using a four-point pain intensity scale. Patients not achieving response within 4 hours were censored to 4 hours.
Figure 1 Estimated Probability of a Patient Responding During the Four Hours Following a Single 2 mg Dose of Dihydroergotamine Mesylate Nasal Spray as a Function of the Time Elapsed Since Initiation of Treatment* 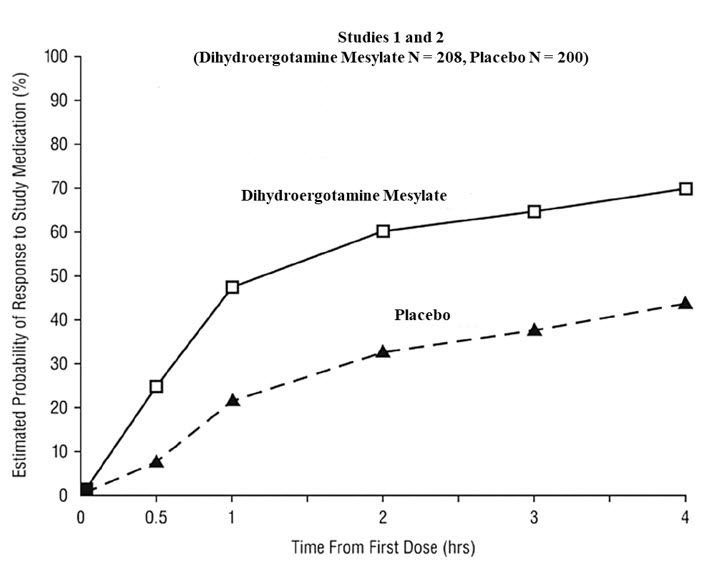
- *
- The figure shows the probability over time of obtaining a response following treatment with dihydroergotamine mesylate nasal spray. Headache response was evaluated on a five-point scale that included pain response. Patients not achieving response within 4 hours were censored to 4 hours.
Figure 2 Estimated Probability of a Patient Responding to Dihydroergotamine Mesylate Nasal Spray During the Four Hours Following Dosing* 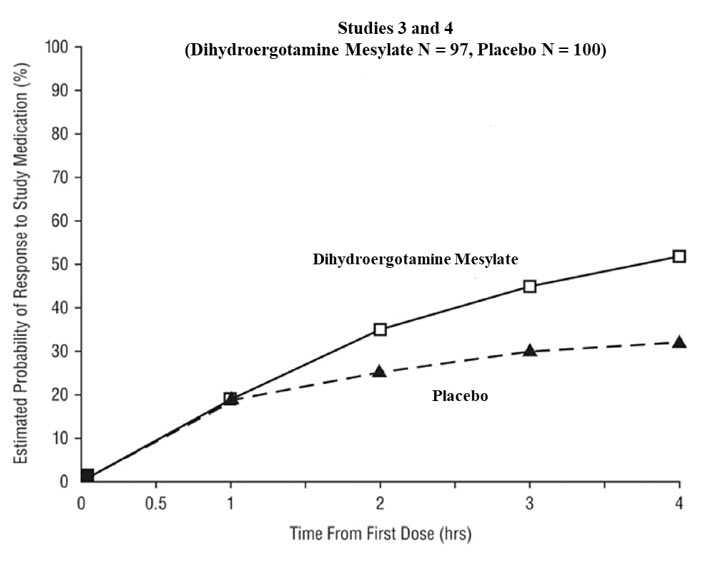
For patients with migraine-associated nausea, photophobia, and phonophobia at baseline, there was a lower incidence of these symptoms at 2 and 4 hours following administration of dihydroergotamine mesylate nasal spray compared to placebo.
Patients were not allowed to use additional treatments for 8 hours prior to study medication dosing and during the 4-hour observation period following study treatment. Following the 4-hour observation period, patients were allowed to use additional treatments. For all studies, the estimated probability of patients using additional treatments for their migraines over the 24 hours following the single 2 mg dose of study treatment is summarized in Figure 3 below.
- *
- Kaplan-Meier plot based on data obtained from all studies with patients not using additional treatments censored to 24 hours. All patients received a single treatment of study medication for their migraine attack. The plot also includes patients who had no response to the initial dose.
Figure 3 Estimated Probability of a Patient Using Additional Treatments for Migraine Over the 24 Hours Following Either Dihydroergotamine Mesylate Nasal Spray 2 mg (or Placebo)* 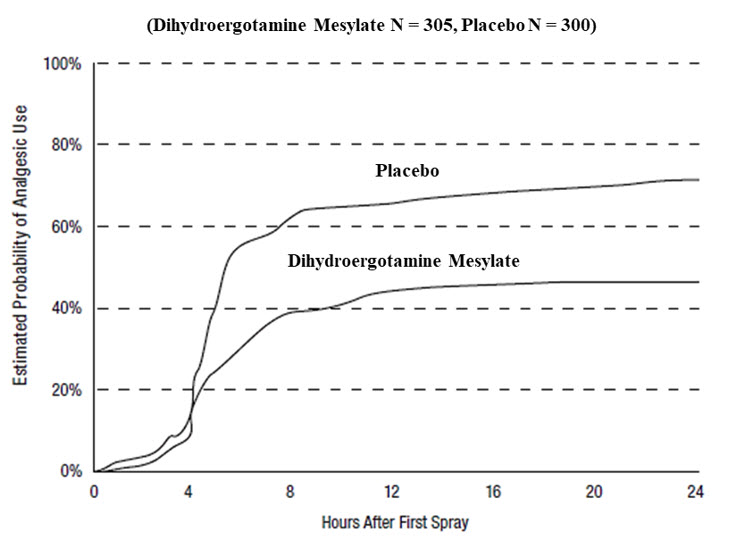
Neither age nor sex appear to affect the patient's response to dihydroergotamine mesylate nasal spray. The racial distribution of patients was insufficient to determine the effect of race on the efficacy of dihydroergotamine mesylate nasal spray.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
TRUDHESA (dihydroergotamine mesylate) nasal spray (0.725 mg per spray) is supplied as a package of 4 single-dose units (NDC 77530-725-04). Each single-dose unit contains:
- One amber glass vial (NDC 77530-725-01) containing 4 mg dihydroergotamine mesylate in a 1 mL clear and colorless to faintly yellow solution. The stopper is not made with natural rubber latex.
- One nasal spray device.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Serious and/or Life-Threatening Reactions with Coadministration of CYP3A4 Inhibitors
Inform patients that serious and/or life-threatening peripheral ischemia (cerebral ischemia and/or ischemia of the extremities) has been associated with the coadministration of dihydroergotamine mesylate and strong CYP3A4 inhibitors, such as macrolide antibiotics and protease inhibitors [see Contraindications (4), Warnings and Precautions (5.1), and Drug Interactions (7.1)].
Myocardial Ischemia and/or Infarction, Other Cardiac Events, Cerebrovascular Events, and Fatalities
Inform patients of the risk for serious cardiac, cerebrovascular, and other vasospasm related events. Advise patients to notify their healthcare provider if they develop any risk factors or symptoms while taking TRUDHESA. Inform patients that nicotine may provoke vasoconstriction predisposing to a greater ischemic response [see Warnings and Precautions (5.2, 5.3, 5.4)].
Medication Overuse Headache
Inform patients that use of drugs to treat migraine attacks for 10 or more days per month may lead to an exacerbation of headache, and encourage patients to record headache frequency and drug use (e.g., by keeping a headache diary) [see Warnings and Precautions (5.6)].
Local Irritation
Advise patients to notify their healthcare provider if they have bothersome local irritation [see Warnings and Precautions (5.9)].
Drug Interactions
Advise patients to inform their healthcare providers if they are taking, or plan to take, any prescription or over-the-counter drugs, since there is a potential for interactions [see Drug Interactions (7)].
Pregnancy
Advise patients of the risk for preterm birth. Advise women to inform their healthcare provider if they are pregnant or intend to become pregnant [see Warnings and Precautions (5.7), Use in Specific Populations (8.1)].
Lactation
Advise patients not to breastfeed during treatment with TRUDHESA [see Use In Specific Populations (8.2)].
Important Administration Instructions
Advise patients that TRUDHESA must be assembled prior to use and that, prior to administration, the device must be primed (i.e., pumped 4 times). Instruct patients to use or discard TRUDHESA within 8 hours once the vial has been opened or the product has been assembled.
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration Issued: 8/2023 MEDICATION GUIDE
TRUDHESA® (true - deh - sa)
(dihydroergotamine mesylate)
nasal sprayWhat is the most important information I should know about TRUDHESA?
TRUDHESA can cause serious side effects, including:- Serious problems with blood circulation to your legs and feet (peripheral ischemia). TRUDHESA can cause peripheral ischemia when you take it with certain medicines known as CYP3A4 inhibitors. Peripheral ischemia may lead to a stroke and death. Stop taking TRUDHESA and get emergency medical help right away if you have any of the following symptoms:
- cramping and pain in your legs or hips
- feeling of heaviness or tightness in your leg muscles
- burning or aching pain in your feet or toes while resting
- numbness, tingling, or weakness in your legs
- cold feeling or color changes in 1 or both legs or feet
- slurred speech
- sudden weakness
- ritonavir
- nelfinavir
- erythromycin
- clarithromycin
- ketoconazole
- itraconazole
These are not all of the medicines that could affect how TRUDHESA works. Your healthcare provider can tell you if it is safe to take TRUDHESA with other medicines. What is TRUDHESA?
TRUDHESA is a prescription medicine used for the acute treatment of migraine with or without aura in adults.- TRUDHESA is not used to prevent migraine.
- TRUDHESA is not used to treat other types of headaches such as hemiplegic (that make you unable to move on one side of your body) or basilar (rare form of migraine with aura) migraines.
Do not take TRUDHESA if you: - are taking medicines known as strong CYP3A4 inhibitors.
- have heart problems or a history of heart problems.
- have uncontrolled high blood pressure.
- have narrowing of blood vessels in your legs, arms, stomach, or kidneys (peripheral vascular disease).
- have sepsis.
- have had vascular surgery.
- have severe liver problems.
- have severe kidney problems.
- are allergic to dihydroergotamine mesylate, ergot alkaloids, or any ingredients in TRUDHESA. See the end of this Medication Guide for a complete list of ingredients.
- have taken any of the following medicines in the last 24 hours:
- sumatriptan
- almotriptan
- eletriptan
- frovatriptan
- naratriptan
- rizatriptan
- ergotamine or ergotamine-type medicines
- have taken any medicines that constrict your blood vessels or raise your blood pressure.
Before you take TRUDHESA, tell your healthcare provider about all of your medical conditions, including if you: - have high blood pressure.
- have liver problems.
- have kidney problems.
- smoke.
- are pregnant or plan to become pregnant. TRUDHESA may cause preterm labor. TRUDHESA should be avoided during pregnancy. Talk to your healthcare provider right away if you are pregnant or want to become pregnant.
- are breastfeeding or plan to breastfeed. TRUDHESA may reduce breast milk supply and pass into your breast milk. TRUDHESA may be harmful to your baby. Do not breastfeed your baby while taking TRUDHESA and for 3 days after you use TRUDHESA. Talk with your healthcare provider about the best way to feed your baby if you take TRUDHESA.
Especially tell your healthcare provider if you take:- sumatriptan
- ergot-type medicine
- saquinavir
- nefazodone
- fluconazole
- grapefruit juice
- zileuton
- nicotine
- propranolol or other medicines that can lower your heart rate
- any medicines that can increase your blood pressure
- selective serotonin reuptake inhibitors
These are not all of the medicines that could affect how TRUDHESA works. Your healthcare provider can tell you if it is safe to take TRUDHESA with other medicines. How should I take TRUDHESA? - Certain people should take their first dose of TRUDHESA in their doctor's office or in another medical setting. Ask your doctor if you should take your first dose in a medical setting.
- Use TRUDHESA exactly as your healthcare provider tells you to use it. Read and follow the instructions in the Instructions for Use which is provided with the TRUDHESA package before using.
- You should use TRUDHESA as soon as the symptoms of your headache start, but it may be given at any time during a migraine.
- After putting TRUDHESA together and priming the device, spray 1 time in each nostril (a complete dose).
- If your headache comes back after the first complete dose or you only get some relief from your headache, you can use a second dose 1 hour after the first complete dose. Use a new TRUDHESA nasal spray device for the second dose.
- Do not use more than 2 doses of TRUDHESA within a 24-hour period or 3 doses within a 7-day period.
- If you use too much TRUDHESA, call your healthcare provider or go to the nearest hospital emergency room right away.
- Taking TRUDHESA for 10 or more days in 1 month may make your headaches worse. You should write down when you have headaches and when you take TRUDHESA so that you can talk with your healthcare provider about how TRUDHESA is working for you.
What are the possible side effects of TRUDHESA?
TRUDHESA can cause serious side effects, including:
See "What is the most important information I should know about TRUDHESA?"-
Heart attack and other heart problems.Heart problems may lead to death. Stop taking TRUDHESA and get emergency medical help right away if you have any of the following symptoms of a heart attack:
- discomfort in the center of your chest that lasts for more than a few minutes, or that goes away and comes back
- severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw
- pain or discomfort in your arms, back, neck, jaw, or stomach
- shortness of breath with or without chest discomfort
- breaking out in a cold sweat
- nausea or vomiting
- feeling lightheaded
- have high blood pressure
- smoke
- have diabetes
- have high cholesterol levels
- are overweight
- have a family history of heart disease
- Stroke. Stop taking TRUDHESA and get emergency medical help right away if you have any of the following symptoms of a stroke:
- face drooping
- slurred speech
- unusual weakness or numbness
- Changes in color or sensation in your fingers and toes (Raynaud's syndrome).
- Stomach and intestinal problems (gastrointestinal and colonic ischemic events). Symptoms of gastrointestinal and colonic ischemic events include:
- sudden or severe stomach pain
- stomach pain after meals
- weight loss
- nausea or vomiting
- constipation or diarrhea
- bloody diarrhea
- fever
- Increase blood pressure.
- Medicine overuse headache. Some people who use too much TRUDHESA may make their headaches worse (medicine overuse headache). If your headaches get worse, your healthcare provider may decide to stop your treatment with TRUDHESA.
- Preterm labor.
- Tissue changes (fibrotic complications). Inflammation and fiber-like tissue that is not normal (fibrosis) can occur around the lungs and stomach.
- Burning feelings in your nose, mouth and throat and abnormal taste.
- runny nose
- nausea
- abnormal taste
- application site reactions
- dizziness
- vomiting
- sleepiness
- sore throat
- diarrhea
These are not all the possible side effects TRUDHESA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store TRUDHESA?
Keep TRUDHESA away from heat and light.- Store TRUDHESA at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not refrigerate or freeze.
- After a TRUDHESA vial has been opened, it must be thrown away after 8 hours.
Do not throw TRUDHESA into fire or incinerators as the canister inside the device may explode.General information about the safe and effective use of TRUDHESA.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use TRUDHESA for a condition for which it was not prescribed. Do not give TRUDHESA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about TRUDHESA that is written for health professionals.What are the ingredients in TRUDHESA?
Active ingredient: Dihydroergotamine mesylate
Inactive ingredients: Caffeine, carbon dioxide, dextrose, and water. The nasal spray device canister contains hydrofluoroalkane-134a (HFA) propellant. The vial stopper is not made with natural rubber latex.
TRUDHESA is a registered trademark of Impel Pharmaceuticals Inc.
Marketed by: Impel Pharmaceuticals Inc., Seattle, WA 98119, USA
www.impelpharma.com
Manufactured by: Mipharm, S.p.A. Milano, Italy
Manufactured for: Impel Pharmaceuticals Inc. 201 Seattle, WA 98119 USA
For more information, go to www.trudhesa.com or call 1-833-878-3437. - Serious problems with blood circulation to your legs and feet (peripheral ischemia). TRUDHESA can cause peripheral ischemia when you take it with certain medicines known as CYP3A4 inhibitors. Peripheral ischemia may lead to a stroke and death. Stop taking TRUDHESA and get emergency medical help right away if you have any of the following symptoms:
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
Trudhesa™
(true-deh-sa)
(dihydroergotamine mesylate)
nasal spray
For Nasal Use Only
622344
Remove and Follow When Dosing
This Instructions for Use contains information on how to deliver Trudhesa.Introduction
Read this Instructions for Use before you start to use Trudhesa and each time you get a prescription refill. There may be new information.
This information does not take the place of talking with your healthcare provider about your medical condition or treatment. You and your healthcare provider should talk about Trudhesa when you start taking it and at regular checkups.
It is important to follow these directions accurately in order to receive the correct dose. Contact your healthcare provider if you have any questions about how to use this product.
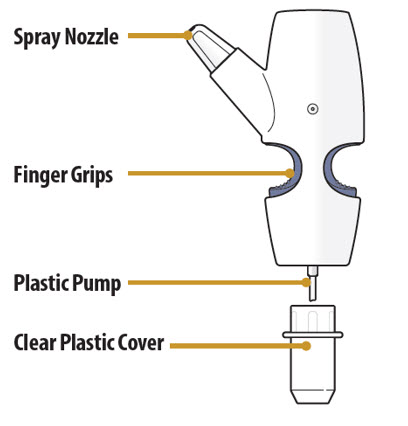
Nasal Spray Device Parts
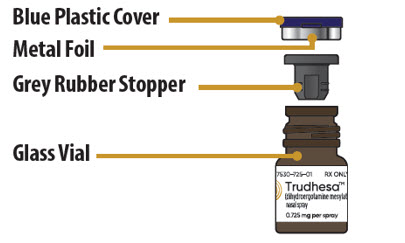
Glass Vial Parts
Important Information You Need To Know Before Dosing with Trudhesa - For nasal use only.
- Always prime the nasal spray device before dosing by pumping the finger grips and glass vial together exactly 4 times.
- The purpose of priming is to bring the medicine to the tip of the spray nozzle. You may or may not see liquid or spray come out of the nozzle during each priming action.
- During priming, make sure to aim the spray nozzle away from your face and anything that you do not want coming into contact with the spray of medicine.
- A complete dose is 2 sprays; 1 spray in each nostril.
- Do not take more than 2 doses within a 24-hour period. Do not take more than 3 doses in a 7-day period.
- Always hold the nasal spray device perfectly upright when priming and when dosing.
- Sniffing while dosing is not necessary. However, sniffing will not hurt you nor make the medicine less effective.
- This nasal spray device product is single-dose (for one complete dose only) and should be thrown away (discarded) after use. You will need a new kit for each dose.
- Keep the product in the case until ready to use.
- After a TRUDHESA vial has been opened, it must be thrown away after 8 hours.
- Do not open the glass vial and expose to air until ready to use.
- Store at room temperature in a clean, dry area.
- Do not use if product is damaged.
- Do not use if product is expired.
- Each glass vial and nasal spray device can only be used 1 time. Throw away the entire nasal spray device after dosing, without removing the glass vial.
- You can take another complete dose at least 1 hour after your first dose if your symptoms persist
Storing Trudhesa
- Store Trudhesa at room temperature between 68 °F to 77°F (20 °C to 25°C).
- Store Trudhesa in the original packaging in a clean, dry area away from heat and light (Figure A).
- Keep Trudhesa in the original packaging until you are ready to use.
- Do not refrigerate or freeze Trudhesa.
- Keep Trudhesa and all medicines out of the reach of children.
Figure A
Preparing to Dose with Trudhesa
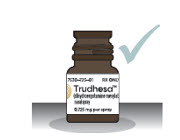
Step 1: Gather and Check Supplies- 1.1
- Check to make sure you are using the right medicine for your migraine (see Figure B). Figure B
- 1.2
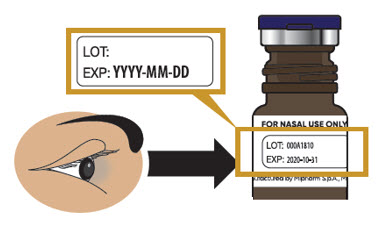
- Check to make sure Trudhesa is not expired (EXP) (see Figure C).
- If expired, throw away and get a new glass vial.
- 1.3
- Check to make sure that the glass vial and blue plastic cover do not look damaged.
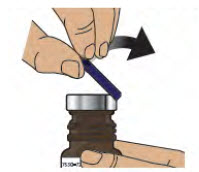
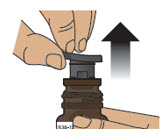
Step 2: Remove Blue Plastic Cover, Metal Foil and Grey Rubber Stopper from Glass Vial - 2.1
- Remove (flip up) the blue plastic cover from the glass vial (see Figure D).
- 2.2
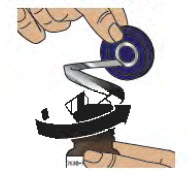
- Use the blue plastic cover to slowly peel away all the metal foil from the grey rubber stopper in a circular motion (see Figure E).
NOTE: The metal foil may come off in 2 or more pieces. Make sure to remove all of the metal foil.
Figure D Figure E - 2.3
- Pull the grey rubber stopper up and out of the glass vial (Figure F and Figure G).
Figure F Figure G - 2.4
- Throw away (discard) cover, foil and grey rubber stopper into the trash.
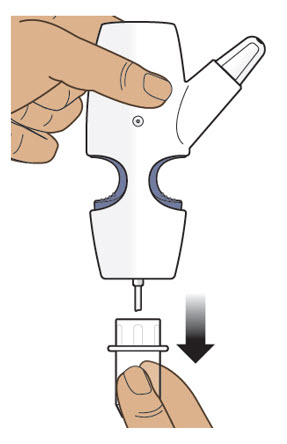
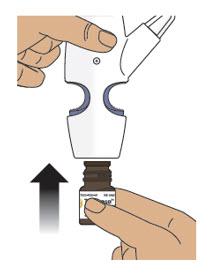
Step 3: Remove the Clear Plastic Cover from the Nasal Spray Device Step 4: Screw the Glass Vial into the Nasal Spray Device - 4.1
- Hold the nasal spray device upright.
- 4.2
- Gently push glass vial into the bottom of the nasal spray device (see Figure I) and screw it on until it is secured as shown in Figure J.
Figure I Figure J Step 5: Prime the Nasal Spray Device by Pumping Four Times with Fin and Thumb - 5.1
- Hold the nasal spray device upright.
- 5.2
- Point the spray nozzle away from your face.
- 5.3
- Place your thumb on the bottom of the glass vial and place your pointer (index) and middle fingers on the finger grips (see Figure K).
- 5.4
- Pump the nasal spray device exactly 4 times.
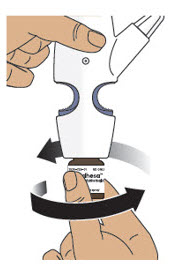
- To pump the nasal spray device, firmly press the finger grips down and press the glass vial up at the same time. Then release (see Figure K).
- You may see some medicine spray out during priming. This is normal. It is okay if you do not see medicine spray out on the first few pumps.
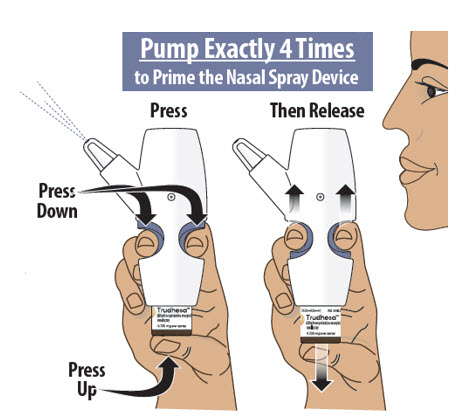
You may or may not see liquid each time you pump.
Only pump 4 times to prime.
Figure KImportant Tip: The purpose of priming is to bring the medicine to the tip of the spray nozzle. If you do not prime the nasal spray device, you will not get your correct dose of medicine.
Always prime the nasal spray device before use by pumping exactly 4 times.
During priming, make sure to aim the nozzle away from your face and anything that you do not want coming into contact with the spray of medicine.Using Trudhesa Step 6: Position the Nasal Spray Device - 6.1
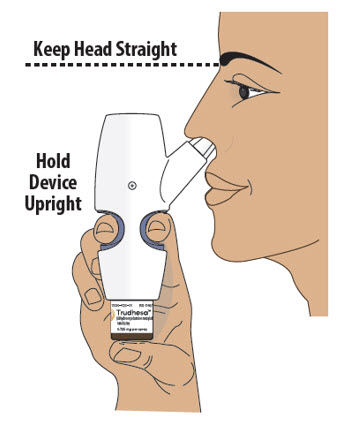
- Turn or re-grip the nasal spray device so that the spray nozzle faces you.
- 6.2
- Make sure your head is straight and the nasal spray device is upright.
- 6.3
- Insert the spray nozzle into your first nostril as far as comfortable (see Figure L).
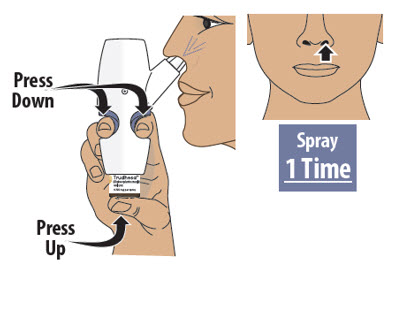
Figure L Step 7: Spray the First Spray into 1 Nostril Step 8: Spray the Second Spray into Other Nostril - 7.1
- Firmly press the finger grips down and press the glass vial up at the same time to deliver the first spray (see Figure M). Then release.
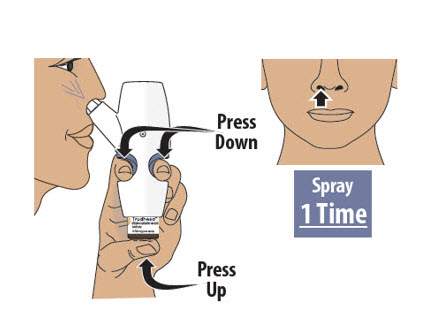
- 8.1
- Move the spray nozzule into your other nostril.
- 8.2
- Firmly press the finger grips down and press the glass vial up at the same time to deliver the second spray (see Figure N). Then release.
Important Tip: A complete dose is 2 sprays; 1 spray in each nostril.
Do not take more than 2 doses within a 24-hour period. Do not take more than 3 doses in a 7-day period. Please refer to the prescribing information for more information.
Sniffing while or after dosing is not necessary. However, sniffing will not hurt you or make the medicine less effective.Step 9: Throw away Trudhesa - Do not separate the glass vial from the nasal spray device.
- Do not reuse the glass vial or the nasal spray device. The glass vial and nasal spray device can only be used 1 time.
- Do not throw Trudhesa into fire or incinerators.
Important Frequently Asked Questions (FAQs)
Question: Can I save medicine by skipping the 4 pumps in "Step 5: Prime the Nasal Spray Device"?
Answer: No. Skipping the 4 pumps to prime the nasal spray device can result in you not getting your correct dose of medicine.
Question: When I first pumped the nasal spray device to prime nothing seemed to happen. Why?
Answer: The purpose of priming is to bring the medicine up to the tip of the nozzle. Even though you may not see or hear anything on your first pump or two, the pumping action will move the medicine from the glass vial through the inside of the nasal spray device and into the nozzle. You should see a spray by your fourth pump attempt. Always prime by pumping exactly 4 times prior to dosing.
Question: Can I reuse the nasal spray device with a new glass vial?
Answer: No. The nasal spray device is for one-time use only and must be thrown away after you have dosed (1 spray in each nostril). This is because the device may clog. After dosing, leave the glass vial screwed onto the nasal spray device and throw away the assembled nasal spray device into the trash. Do not recycle any part of the product.
Question: Can I use the medicine that remains in the glass vial for a later dose?
Answer: No. Although it is normal for some medicine to remain in the glass vial, it can not be used for later dosing. Any leftover medicine will become ineffective.
Question: What happens if I spray more than one time in the same nostril?
Answer: A complete and correct dose is one spray into each nostril. Do not dose in your other nostril if you already sprayed two times in one nostril. Talk to your healthcare provider if you have moderate to severe irritation in your nose or changes in smell.
Question: How soon can I take another dose if I am not getting relief from my migraine?
Answer: You can take another dose at least 1 hour after your first dose if your symptoms persist. Do not take more than 2 doses within a 24-hour period. Do not take more than 3 doses in a 7-day period. Please refer to the prescribing information for more information.This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 9/2021 -
PRINCIPAL DISPLAY PANEL - 0.725 mg Vial Carton
Trudhesa™
(dihydroergotamine mesylate) nasal spray0.725 mg per spray
Recommended Dosage: See Prescribing Information
Contains: 4 Nasal Spray Devices, 4 Trudhesa Vials,
Instructions for Use and Prescribing Information
NOT FOR INDIVIDUAL SALE
Contents are disposable; discard used nasal spray device
and vial after use. For nasal use only.RX ONLY
622338

-
INGREDIENTS AND APPEARANCE
TRUDHESA
dihydroergotamine mesylate spray, meteredProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:77530-725 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dihydroergotamine mesylate (UNII: 81AXN7R2QT) (DIHYDROERGOTAMINE - UNII:436O5HM03C) Dihydroergotamine mesylate 4 mg in 1 mL Inactive Ingredients Ingredient Name Strength Caffeine (UNII: 3G6A5W338E) Anhydrous Dextrose (UNII: 5SL0G7R0OK) Carbon Dioxide (UNII: 142M471B3J) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:77530-725-04 4 in 1 CARTON 09/24/2021 1 1 mL in 1 VIAL, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:77530-725-00 1 in 1 CARTON 09/24/2021 2 1 mL in 1 VIAL, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213436 09/24/2021 Labeler - Impel Pharmaceuticals Inc. (827915385)