Label: PROPOFOL LIPURO injection, emulsion
-
Contains inactivated NDC Code(s)
NDC Code(s): 0264-4850-01 - Packager: B. Braun Medical Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 20, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
FACT SHEET FOR HEALTH CARE PROVIDERS
EMERGENCY USE AUTHORIZATION (EUA) OF PROPOFOL-LIPURO 1%
INJECTABLE EMULSION FOR INFUSION
The U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) to permit the emergency use of the unapproved product, Propofol-Lipuro 1% injectable emulsion for infusion in 100 mL to maintain sedation via continuous infusion in patients greater than 16 years old who require mechanical ventilation in an intensive care unit (ICU) setting.
Propofol-Lipuro 1% injectable emulsion for infusion is not an FDA-approved drug in the United States. However, FDA has issued an EUA permitting the emergency use of Propofol-Lipuro 1% injectable emulsion for infusion during the COVID-19 pandemic and related shortage of propofol drug product.
Propofol-Lipuro 1% injectable emulsion for infusion is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3-(b)(1), unless the authorization is terminated or revoked sooner.
The scope of the EUA is limited as follows:
- Propofol-Lipuro 1% injectable emulsion for infusion will be used only to maintain sedation via continuous infusion in patients greater than 16 years old who require mechanical ventilation.
- Propofol-Lipuro 1% injectable emulsion for infusion will be administered only by a licensed healthcare provider in an ICU setting.
- Propofol-Lipuro 1% injectable emulsion for infusion will NOT be administered to pregnant women, unless there are no FDA-approved products available to maintain sedation for these patients should they require mechanical ventilation in an ICU setting.
- Propofol-Lipuro 1% injectable emulsion for infusion will be used only in accordance with the dosing regimens as detailed in the authorized Fact Sheets.
Product Description
Consistent with the EUA, B. Braun Melsungen AG, Germany, will offer the following presentations of Propofol-Lipuro 1% Emulsion.
Product Name And Description MCT/LCT Concentration Source/Type of Oil Size National Drug Code (NDC) Propofol-Lipuro 1 % injectable emulsion for infusion 1,000 mg in 100 mL (propofol 10 mg per mL) Medium Chain Triglycerides (MCT) 50 mg/mL Long Chain Triglycerides (LCT) 50 mg/mL
Soybean oil, refined; medium-chain triglycerides 100 mL NDC 0264-4850-01 Propofol-Lipuro 1% injectable emulsion for infusion is approved in Europe as well as in many other international countries.
Propofol-Lipuro 1% injectable emulsion for infusion will be manufactured by B. Braun facilities in Germany as Propofol-Lipuro 1% Emulsion supplied in all other countries worldwide. The B. Braun’s manufacturing sites are inspected regularly by German and other National Competent Authorities confirming the fulfillment of good manufacturing practices (GMP) and other current standards. The manufacturing site in Melsungen, Germany was previously inspected by FDA.
Key Differences between FDA-approved Diprivan (propofol) Injectable Emulsion, USP Products and Propofol-Lipuro 1% (Propofol) injectable emulsion for infusion Diprivan (propofol) Injectable Emulsion, USP
Propofol 1,000 mg per 100 mL
(10 mg per mL)Propofol-Lipuro 1%(propofol) injectable emulsion for infusion
1,000 mg in
100 mL
(10 mg/mL)What does this mean to you as a healthcare professional? Composition Contains long-chain triglycerides (LCT) Contains a combination of medium-chain triglycerides (MCT) and long-chain triglycerides (LCT) Prolonged IV infusion of MCT to pregnant rabbits has been reported in the published literature to increase the RISK OF NEURAL TUBE DEFECTS.
Because it is not yet clear if there is differential risk for adverse developmental effects with Propofol-Lipuro 1% compared to Diprivan (propofol), Propofol-Lipuro 1% SHOULD NOT BE USED IN PREGNANT WOMEN unless there are no FDA-approved products available to maintain sedation in these patients who require mechanical ventilation in an ICU setting.
Indication General anesthesia, procedural sedation, ICU sedation ICU sedation ONLY Propofol-Lipuro 1% is only to maintain sedation via continuous infusion in patients greater than 16 years old who require mechanical ventilation in the ICU setting. Patient Population Greater than 3 years old (procedural sedation and general anesthesia) Greater than 16 years old (ICU sedation)
Greater than
16 years old
(ICU sedation)Propofol-Lipuro 1% is only indicated to maintain sedation via continuous infusion for patients greater than 16 years old who require mechanical ventilation in the ICU setting. Propofol-Lipuro 1% should not be used in pregnant women unless there are no FDA-approved products available to maintain sedation for these patients who require mechanical ventilation in an ICU setting.
Dosing See package insert* Administration rates of 0.3 to
4.0 mg propofol/kg
bodyweight/h have been demonstrated to provide adequate sedationInfusion rates greater than 4.0 mg propofol/kg bodyweight/h are not recommended due to risk of Propofol Infusion Syndrome.
The duration of administration must not exceed 7 days.
Method of Administration Bolus or infusion Infusion ONLY Propofol-Lipuro 1% should be administered undiluted intravenously by continuous infusion. DO NOT ADMINISTER PROPOFOL-LIPURO 1% VIA BOLUS INJECTION. Containers should be shaken before use. If two layers can be seen after shaking, the emulsion should not be used.
Do not admix with other medicinal products. Co-administration of other medicinal products or fluids added to the Propofol-Lipuro 1% infusion line must occur close to the cannula site using a Y-piece connector or a three-way valve. Propofol-Lipuro 1% Emulsion must not be administered via a microbiological filter.
Other Special Patient Populations See package insert* Caution should be
taken when treating
patients with
mitochondrial disease,
epilepsy, and disorders
of fat metabolism.Patients with mitochondrial disease may be susceptible to exacerbations of their disorder when undergoing ICU care. Maintenance of normothermia, provision of carbohydrates and good hydration are recommended for such patients. The early presentations of mitochondrial disease exacerbation and of the ‘propofol infusion syndrome’ may be similar. Although several studies have demonstrated efficacy in treating status epilepticus, administration of propofol in epileptic patients may also increase the risk of seizure. Prior to administration of Propofol‐Lipuro 1%, antiepileptic medication(s) should be administered to patients with a history of medically managed seizures or when clinically indicated. For these patients, as well as for ARDS/respiratory failure and tetanus patients, sedation maintenance dosages were generally higher than those for other critically ill patient populations.
Appropriate care should be applied in patients with disorders of fat metabolism and in other conditions where lipid emulsions must be used cautiously. It is recommended that blood lipid levels should be monitored if propofol is administered to patients thought to be at particular risk of fat overload. Administration of propofol should be adjusted appropriately if the monitoring indicates that fat is being inadequately cleared from the body. If the patient is receiving other intravenous lipid concurrently, a reduction in quantity should be made in order to take account of the amount of lipid infused as part of the propofol formulation; 1.0 mL of Propofol-Lipuro 1 % Emulsion contains approximately 0.1 g of fat.
Drug interaction See package insert* Drug interaction with rifampicin, valproate Profound hypotension has been reported following anesthetic induction with propofol in patients treated with rifampicin. A need for lower propofol doses has been observed in patients taking valproate. When used concomitantly, a dose reduction of propofol may be considered.
Presence of antimicrobial retardant Yes NO Propofol-Lipuro 1 % does NOT contain an antimicrobial retardant and supports growth of microorganisms. STRICT ASEPTIC TECHNIQUE MUST ALWAYS BE MAINTAINED DURING HANDLING.
Each vial of Propofol-Lipuro 1 % is intended only for single administration for an individual patient. Vials are not intended for multiple use.
Propofol-Lipuro 1% must be drawn up aseptically into a sterile syringe or an infusion set immediately after breaking the vial seal. Administration must commence without delay. Asepsis must be maintained for both Propofol-Lipuro 1 % Emulsion and the infusion equipment throughout the infusion period.
The unused portion of a vial should be discarded immediately after opening. As with any propofol used in infusion, discard all product and infusion lines after 12 hours.
Contraindications See package insert* Propofol-Lipuro 1 % Emulsion should not be used in patients who are hypersensitive to peanut or soy Propofol-Lipuro 1 % is contraindicated in patients with a known hypersensitivity to the active substance or to any of the excipients: soybean oil, refined; medium-chain triglycerides; glycerol; egg lecithin; sodium oleate; water for injections.
Propofol-Lipuro 1 % contains soya-bean oil and should not be used in patients who are hypersensitive to peanut or soy.
Bar code Unit of use barcode on individual vials No unit of use barcode The barcode on the imported product label may not register accurately with the U.S. scanning systems. Institutions should manually input the imported product information into their systems and confirm that the barcode, if scanned, provides correct information. Alternative procedures should be followed to assure that the correct drug product is being used and administered to individual patients. *Refer to https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019627s066lbl.pdf for the Diprivan package insert.
Please refer to the package insert for the full prescribing information of Propofol-Lipuro 1% injectable emulsion for infusion.
For questions regarding Propofol-Lipuro 1% injectable emulsion for infusion, please contact B. Braun Medical Inc.:
Company Name: B. Braun Medical Inc.
Address: 861 Marcon Blvd, Allentown, PA 18109
Country: United States
24-hour Telephone: +1 833-425-1464
E-Mail: productqualityexcellence@bbraunusa.comWhat is an EUA
The United States FDA has made Propofol-Lipuro 1% injectable emulsion for infusion available to treat patients in an ICU during the COVID-19 pandemic under an emergency access mechanism called an Emergency Use Authorization (EUA). This EUA is supported by a Secretary of Health and Human Service (HHS) declaration that circumstances exist to justify the emergency use of drugs and biological products during the COVID-19 pandemic.
Propofol-Lipuro 1% injectable emulsion for infusion made available under an EUA has not undergone the same type of review as an FDA-approved product. FDA may issue an EUA when certain criteria are met, which includes that there are no adequate, approved, available alternatives. Based on the totality of scientific evidence available, it is reasonable to believe that Propofol-Lipuro 1% injectable emulsion for infusion has met certain criteria for safety, performance, and labeling and may be effective to maintain sedation via continuous infusion in patients greater than 16 years old who require mechanical ventilation in an ICU setting.
This EUA for Propofol-Lipuro 1% injectable emulsion for infusion is in effect for the duration of the COVID-19 declaration justifying emergency use of the product, unless terminated or revoked. The EUA will end when the declaration is terminated or revoked or when there is a change in the approval status of the product such that an EUA is no longer needed.
This communication and product information is available on the B. Braun Medical Inc. website https://www.bbraunusa.com/en/company/newsroom/covid19.html# as well as the FDA webpage which includes links to patient fact sheet.
Adverse Event Reporting
Healthcare facilities and prescribing healthcare providers or their designee receiving Propofol-Lipuro 1 % injectable emulsion for infusion will track all medication errors associated with the use of and all serious adverse events that are considered potentially attributable to Propofol-Lipuro 1% injectable emulsion for infusion.
Adverse events or quality problems experienced with the use of this product must also be reported to the FDA using one of the following methods:
- Complete and submit a MedWatch form online: www.fda.gov/medwatch/report.htm or
- Complete and submit FDA Form 3500 (health professional) by fax (1-800-FDA-0178) (this form can be found via link above).
Call 1-800-FDA-1088 for questions. Submitted reports should state, “Propofol-Lipuro 1% injectable emulsion for infusion use for COVID-19 under Emergency Use Authorization (EUA)” at the beginning of the question “Describe Event” for further analysis.
For questions regarding Propofol-Lipuro 1% (10 mg/mL) injectable emulsion for infusion for continuous infusion, please contact B. Braun Medical Inc.:
Company Name: B. Braun Medical Inc.
24-hour Telephone: +1 833-425-1464
E-Mail: productqualityexcellence@bbraunusa.comComparison Table of FDA-approved Diprivan (propofol) Injectable Emulsion, USP 10 mg/mL and
Propofol-Lipuro 1 % (10 mg/mL) injectable emulsion for infusionProduct Name Diprivan (propofol) Injectable Emulsion, USP Propofol-Lipuro 1 %
injectable emulsion for infusionPropofol concentration 10 mg/mL 10 mg/mL Product labels
100 mL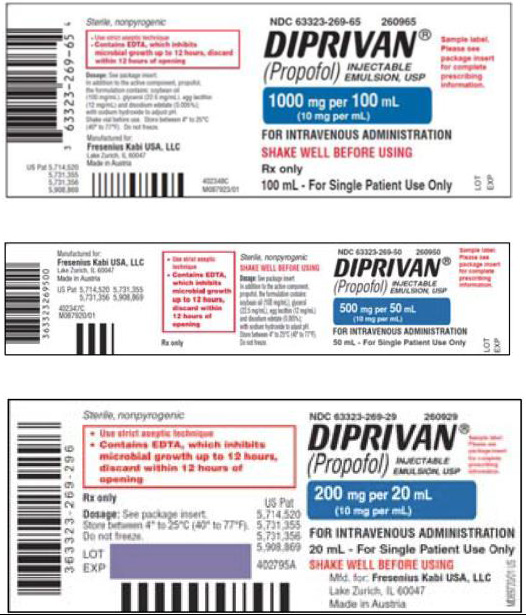

Note: The imported product labels include that Propofol-Lipuro
1% is “for injection or infusion”. However, Propofol-Lipuro 1%
is only authorized for use in the U.S. as “for infusion”.Active Ingredient Propofol Propofol Excipients Soybean oil
Glycerol
Egg phospholipids
Edetate disodium
Sodium hydroxideSoybean oil, refined
Medium-chain triglycerides
Egg phospholipids for injection
Glycerol
Sodium oleate
Water for injectionFill Volume 20 mL
50 mL
100 mL100 mL Duration Drug holiday after 5 days to replace urine zinc losses Do not administer for more than 7 days. Dilution Dilution to 2 mg/mL with
5% Dextrose Injection onlyDo Not Dilute. Bolus Bolus injection permitted Infusion ONLY Description Single Dose Vial for Single Patient Use Only Single Dose Vial for Single Patient Use Only Company Fresenius Kabi USA B. Braun Melsungen AG Germany Propofol-Lipuro 1% (propofol)
injectable emulsion for infusion
1,000 mg in 100 mL (10 mg/mL)

Propofol-Lipuro 1% (propofol) injectable emulsion for infusion 1,000 mg in 100 mL (10 mg/mL) contains the same active ingredient, strength, and concentration as Diprivan® (propofol) injectable emulsion for infusion USP 1,000 mg per 100 mL (10 mg per mL).
- Propofol-Lipuro 1% injectable emulsion for infusion is not FDA-approved
- Propofol-Lipuro 1% injectable emulsion for infusion is approved in Europe, as well as many other international countries outside of the United States.
- Propofol-Lipuro 1% injectable emulsion for infusion has been authorized by FDA for use under an Emergency Use Authorization (EUA)
- Propofol-Lipuro 1% injectable emulsion for infusion is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3-(b)(1), unless the authorization is terminated or revoked sooner
To place an order, contact your local sales representative.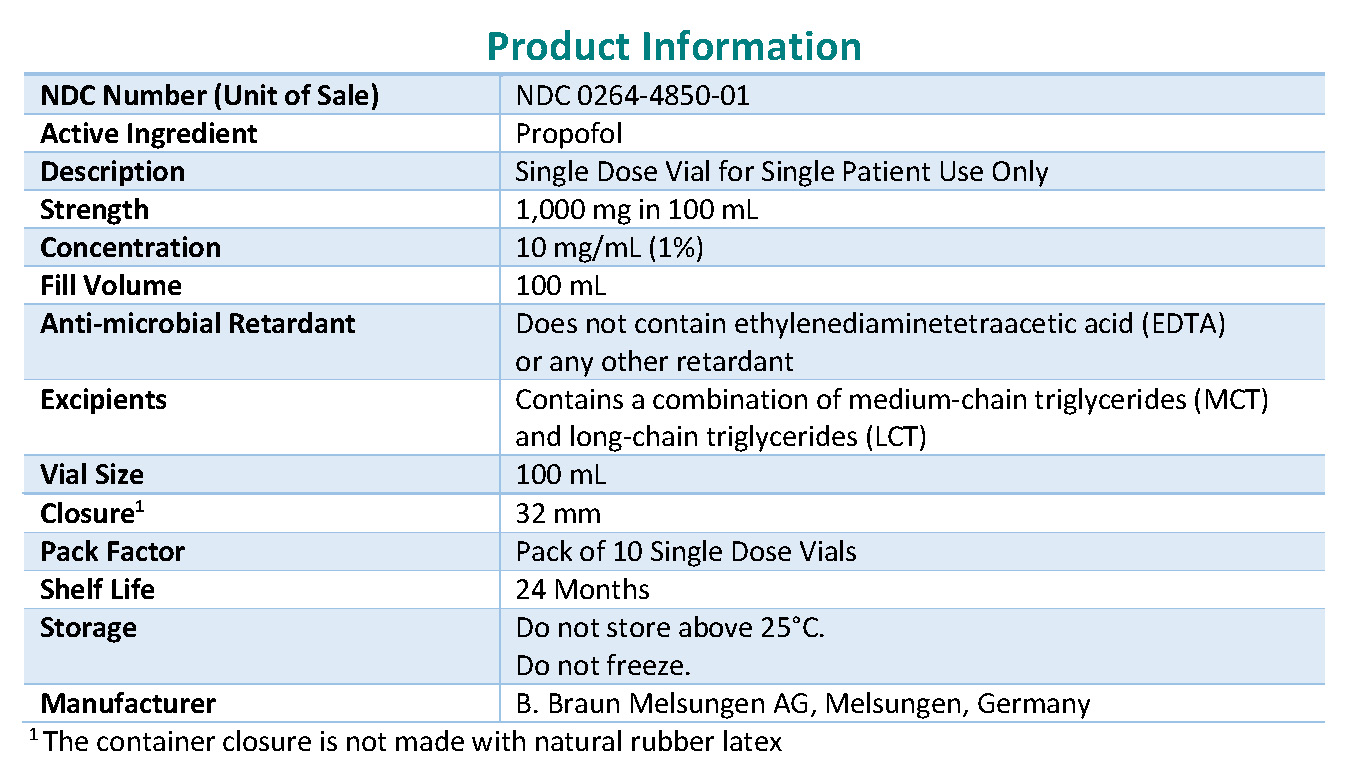
-
Fact Sheet for Patients, Parents, And Caregivers
Emergency Use Authorization (EUA) of PROPOFOL-LIPURO 1% (propofol)
INJECTABLE EMULSION FOR INFUSION
During CORONAVIRUS DISEASE 2019 (COVID-19) Pandemic
This fact sheet contains information to help you understand the risks and benefits of Propofol-Lipuro 1% (10 mg/mL) injectable emulsion for infusion that you have received or may receive.
There is currently a shortage of U.S. Food and Drug Administration (FDA)-approved propofol products that maintain sedation for patients who are on a machine that helps with breathing (ventilator) due to the COVID-19 pandemic. Propofol-Lipuro 1% injectable emulsion for infusion is not an FDA-approved medicine in the United States. Propofol-Lipuro 1% injectable emulsion for infusion contains the same active ingredient, propofol, at the same strength as Diprivan Injectable Emulsion and other propofol products approved in the United States. Propofol-Lipuro 1% injectable emulsion for infusion is currently approved in Europe and other international countries. Read this Fact Sheet for information about Propofol-Lipuro 1% injectable emulsion for infusion. Talk to your healthcare provider if you have questions. It is your choice to take Propofol-Lipuro 1% injectable emulsion for infusion or stop it at any time.
What is COVID-19?
COVID-19 is caused by a virus called a coronavirus. This type of coronavirus has not been seen before. This new coronavirus was first found in people in December 2019. You can get COVID-19 through contact with another person who has the virus.
COVID-19 illnesses have ranged from very mild (including some with no reported symptoms) to severe, including illness resulting in death. While information so far suggests that most COVID-19 illness is mild, serious illness can happen and may cause some of your other medical conditions to become worse. Older people and people of all ages with severe, long lasting (chronic) medical conditions like heart disease, lung disease, and diabetes, for example, seem to be at higher risk of being hospitalized for COVID-19.
The symptoms of COVID-19 are fever, cough and shortness of breath, which may appear 2 to 14 days after exposure. Serious illness including breathing problems can occur and may cause your other medical conditions to become worse.
What is PROPOFOL-LIPURO 1% INJECTABLE EMULSION FOR INFUSION?
Propofol-Lipuro 1% injectable emulsion for infusion belongs to a group of medicines called sedatives/hypnotics. It will be used to help calm (sedate) you if you need a tube inserted (intubation) and a machine to help you breathe (ventilator) while in an ICU.
What do I need to know before I receive PROPOFOL-LIPURO 1% INJECTABLE EMULSION FOR INFUSION?
Who should not receive Propofol-Lipuro 1% injectable emulsion for infusion?
Do not receive Propofol-Lipuro 1% injectable emulsion for infusion if:
- You have received propofol before and have had an allergic reaction to it
- You are allergic (hypersensitive) to soy, peanut, or any of the other ingredients of this medicine
- You are 16 years of age or younger
What should I tell my healthcare provider before I receive Propofol-Lipuro 1% injectable emulsion for infusion?
Please tell your healthcare provider about all of your medical conditions, including if you have:
- Serious head injuries
- Mitochondrial disease
- A disorder in which your body does not handle fat properly or any other health problems in which
giving you fat emulsions may cause a health problem - Dehydration (your blood volume is too low, or hypovolemia)
- Heart, kidney, or liver problems
- High pressure within your brain
- Problems with your breathing
- Epilepsy
- Are pregnant or plan to become pregnant.
- Are breastfeeding or plan to breastfeed
- Are taking any medicines, including prescription, over‐the‐counter, vitamins, or herbal products
Your healthcare provider will consider that other medicines with an inhibiting effect on the central nervous system may increase the effects of propofol when given together with propofol. Special care will be taken if you are also receiving an antibiotic containing rifampicin or an anti-seizure medication containing valproate.
How will I receive PROPOFOL-LIPURO 1% INJECTABLE EMULSION FOR INFUSION?
Propofol-Lipuro 1% injectable emulsion for infusion is given to you through a vein (IV) under the direct supervision of an anesthesiologist or intensive care doctor who will closely control the amount of Propofol-Lipuro 1% injectable emulsion given to you.
Dosage
The dose you are given will vary depending on your age, body weight, and physical condition. The doctor will give the correct dose to achieve the required level of sedation, by carefully watching your responses and vital signs (pulse, blood pressure, breathing, etc).
Propofol-Lipuro 1% injectable emulsion will be given by infusion and only for a maximum of 7 days.
What are the important possible side effects of PROPOFOL-LIPURO 1% INJECTABLE EMULSION FOR INFUSION?
The most common side effects are:
- Pain at the injection site
- Drop in blood pressure
- Changes in heart rate
- Changes in breathing, coughing, and hiccups
- Headache, nausea, or vomiting during recovery from propofol sedation
Less common side effects are:
- Blood clots in veins or inflammation of veins at the injection site
- Seizures
- Fever
- Allergic reactions, which can include swelling of the face, tongue and/or throat, wheezing and/or
difficulty breathing, skin redness, and low blood pressure - Fluid on lungs (lung edema)
- Inflammation of the pancreas
- Loss of sexual control during the time of recovery
- Change in color of your urine after prolonged intravenous administration of propofol
- Skin or tissue damage if the medicine is accidentally injected outside of a vein
- Involuntary movements
- Mood changes
- Drug abuse and drug dependence
- Cardiac arrest, heart failure
- Shallow breathing
- Pain and/or swelling at the injection site after the medicine was accidentally injected outside of a
vein
What other treatment choices are there?
Your anesthesiologist or intensive care doctor may give you other sedation agents depending on your medical condition.
What if I am pregnant or breastfeeding?
Tell your healthcare provider if you are pregnant, think you may be pregnant, or are planning to have a baby. Propofol-Lipuro 1% injectable emulsion for infusion may harm your unborn baby. Propofol-Lipuro 1% injectable emulsion for infusion should be used in pregnant women only if there are no other FDA-approved medicines available for your medical condition.
Propofol-Lipuro 1% injectable emulsion for infusion may pass into breast milk. You should stop breastfeeding and throw away (discard) breast milk for 24 hours after you have received Propofol-Lipuro 1% injectable emulsion for infusion.
How do I report side effects with PROPOFOL-LIPURO 1% INJECTABLE EMULSION FOR INFUSION?
Tell your healthcare provider right away if you have any side effect that bothers you or does not go away.
Report side effects to FDA MedWatch at www.fda.gov/medwatch or call 1-800-FDA-1088. You may also report the problem to the B. Braun Medical Inc. USA by phone at 1-833-425-1464 or by email at productqualityexcellence@bbraunusa.com.
HOW CAN I LEARN MORE ABOUT COVID-19?
- Ask your healthcare provider
- Visit https://www.cdc.gov/COVID19
- Contact your local or state public health department
WHAT IS AN EMERGENCY USE AUTHORIZATION (EUA)?
The United States FDA has made Propofol-Lipuro 1% injectable emulsion for infusion available under an emergency access mechanism called an EUA. The EUA is supported by a Secretary of Health and Human Service (HHS) declaration that circumstances exist to justify the emergency use of drugs and biological products during the COVID-19 pandemic.
Propofol-Lipuro 1% injectable emulsion for infusion has not undergone the same type of review as an FDA-approved product. FDA may issue an EUA when certain criteria are met, which includes that there are no adequate, approved, and available alternatives. In addition, the FDA decision is based on the totality of scientific evidence available showing that it is reasonable to believe that the product meets certain criteria for safety, performance, and labeling, and may be effective in treatment of patients during the COVID-19 pandemic. All of those criteria must be met to allow for the product to be used in the treatment of patients during the COVID-19 pandemic.
The EUA for Propofol-Lipuro 1% injectable emulsion for infusion is in effect for the duration of the COVID-19 declaration justifying emergency use of these products, unless terminated or revoked (after which the products may no longer be used).
-
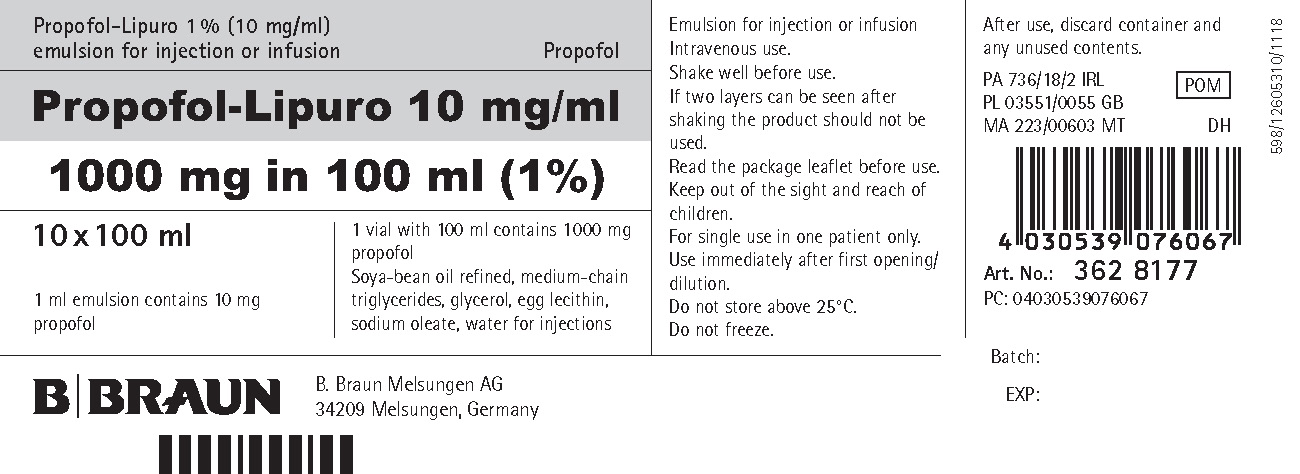
PRINCIPAL DISPLAY PANEL - VIAL
Propofol-Lipuro 1 % (10 mg/ml) emulsion for injection or infusion
Propofol
Propofol-Lipuro 10 mg/ml
1000 mg in 100 ml (1%)1 ml emulsion contains 10 mg propofol
100 ml emulsion contain 1 000 mg propofolSoya-bean oil refined, medium-chain triglycerides, glycerol, egg lecithin, sodium oleate, water for injections
Emulsion for injection or infusion Intravenous use. Shake well before use. Read the package leaflet before use. Keep out of the sight and reach of children.
For single use in one patient only. Use immediately after first opening/ dilution. Do not store above 25°C. Do not freeze. After use, discard container and any unused contents.
PA 736/18/2 IRL
POM
PL 03551/0055 GB; MA 223/00603 MT DH
B. Braun Melsungen AG
34209 Melsungen, Germany598/12299820/0819
Batch:
EXP:

-
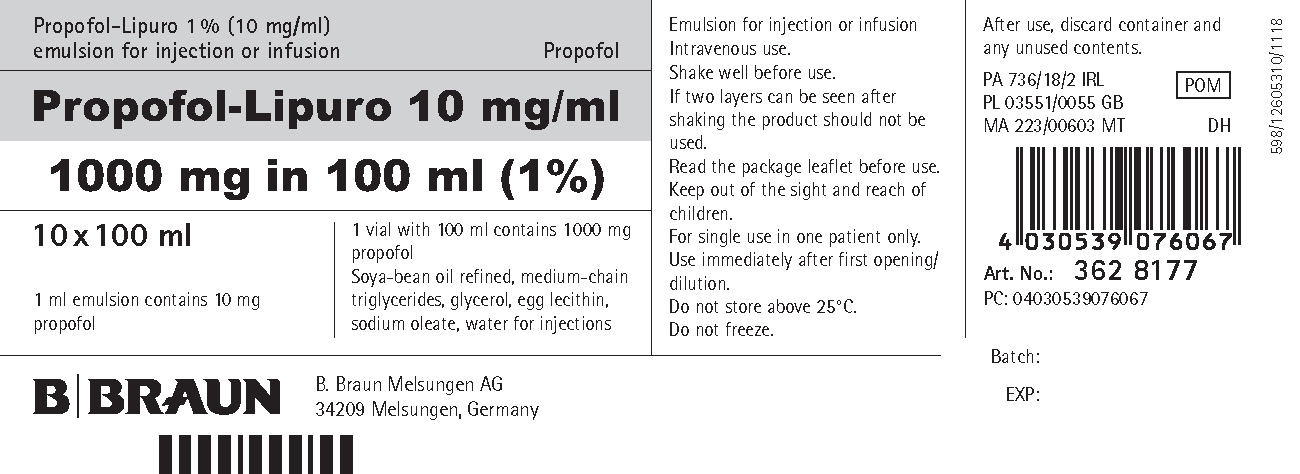
PRINCIPAL DISPLAY PANEL - CARTON
Propofol-Lipuro 1 % (10 mg/ml)
emulsion for injection or infusionPropofol
Propofol-Lipuro 10 mg/ml
1000 mg in 100 ml (1%)10 x 100 ml
1 ml emulsion contains 10 mg propofol
1 vial with 100 ml contains 1 000 mg propofol
Soya-bean oil refined, medium-chain triglycerides, glycerol, egg lecithin, sodium oleate, water for injectionsEmulsion for injection or infusion Intravenous use.
Shake well before use.
If two layers can be seen after shaking the product should not be used.
Read the package leaflet before use. Keep out of the sight and reach of children.
For single use in one patient only. Use immediately after first opening/ dilution.
Do not store above 25°C.
Do not freeze.After use, discard container and any unused contents.
PA 736/18/2 IRL
PL 03551/0055 GB
MA 223/00603 MT DH
POM
Art. No.: 362 8177
PC: 04030539076067
B. Braun Melsungen AG
34209 Melsungen, Germany598/12605310/1118
Batch:
EXP:
-
INGREDIENTS AND APPEARANCE
PROPOFOL LIPURO
propofol lipuro injection, emulsionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0264-4850 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPOFOL (UNII: YI7VU623SF) (PROPOFOL - UNII:YI7VU623SF) PROPOFOL 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SODIUM OLEATE (UNII: 399SL044HN) WATER (UNII: 059QF0KO0R) SOYBEAN OIL (UNII: 241ATL177A) GLYCERIN (UNII: PDC6A3C0OX) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0264-4850-01 10 in 1 CARTON 03/12/2021 1 100 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/12/2021 Labeler - B. Braun Medical Inc. (002397347)