Label: RYBREVANT- amivantamab injection
- NDC Code(s): 57894-501-00, 57894-501-01
- Packager: Janssen Biotech, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RYBREVANT safely and effectively. See full prescribing information for RYBREVANT.
RYBREVANT ®(amivantamab-vmjw) injection, for intravenous use
Initial U.S. Approval: 2021RECENT MAJOR CHANGES
INDICATIONS AND USAGE
RYBREVANT is a bispecific EGF receptor-directed and MET receptor-directed antibody indicated:
- in combination with carboplatin and pemetrexed for the first-line treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, as detected by an FDA-approved test. ( 1, 2.2)
- as a single agent for the treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as detected by an FDA-approved test, whose disease has progressed on or after platinum-based chemotherapy. ( 1, 2.2)
DOSAGE AND ADMINISTRATION
- The recommended dosage of RYBREVANT is based on baseline body weight and administered as an intravenous infusion after dilution. ( 2.3, 2.5, 2.6, 2.7)
- Administer premedications as recommended. ( 2.4)
- Administer via a peripheral line on Week 1 and Week 2. ( 2.7)
- Administer RYBREVANT in combination with chemotherapy weekly for 4 weeks, with the initial dose as a split infusion in Week 1 on Day 1 and Day 2, then administer every 3 weeks starting at Week 7. ( 2.3)
- Administer RYBREVANT as a single agent weekly for 4 weeks, with the initial dose as a split infusion in Week 1 on Day 1 and Day 2, then administer every 2 weeks starting at Week 5. ( 2.4)
- Administer diluted RYBREVANT intravenously according to the infusion rates in Tables 7 and 8. ( 2.8)
Body Weight (at Baseline) Dosage Recommended Dose RYBREVANT in Combination with Carboplatin and Pemetrexed Less than 80 kg Weeks 1–4 1400 mg Week 7 onwards 1750 mg Greater than or equal to 80 kgs Weeks 1–4 1750 mg Week 7 onwards 2100 mg RYBREVANT as a Single Agent Less than 80 kg Weeks 1–5
Week 7 onwards1050 mg Greater than or equal to 80 kg Weeks 1–5
Week 7 onwards1400 mg DOSAGE FORMS AND STRENGTHS
Injection: 350 mg/7 mL (50 mg/mL) solution in a single-dose vial ( 3)
CONTRAINDICATIONS
None. ( 4)
WARNINGS AND PRECAUTIONS
- Infusion-Related Reactions (IRR): Interrupt infusion at the first sign of IRRs. Reduce infusion rate or permanently discontinue RYBREVANT based on severity. ( 2.5, 5.1)
- Interstitial Lung Disease (ILD)/Pneumonitis: Monitor for new or worsening symptoms indicative of ILD. Immediately withhold RYBREVANT in patients with suspected ILD/pneumonitis and permanently discontinue if ILD/pneumonitis is confirmed. ( 2.5, 5.2)
- Dermatologic Adverse Reactions: May cause rash including acneiform dermatitis and toxic epidermal necrolysis. Withhold, dose reduce or permanently discontinue RYBREVANT based on severity. ( 2.5, 5.3)
- Ocular Toxicity: Promptly refer patients with worsening eye symptoms to an ophthalmologist. Withhold, dose reduce or permanently discontinue RYBREVANT based on severity. ( 2.5, 5.4)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to the fetus and to use effective contraception. ( 5.5, 8.1, 8.3)
ADVERSE REACTIONS
RYBREVANT in Combination with Carboplatin and Pemetrexed
- The most common adverse reactions (≥ 20%) were rash, nail toxicity, stomatitis, infusion-related reaction, fatigue, edema, constipation, decreased appetite, nausea, COVID-19, diarrhea, and vomiting. ( 6.1)
- The most common Grade 3 or 4 laboratory abnormalities (≥ 2%) were decreased albumin, increased alanine aminotransferase, increased gamma-glutamyl transferase, decreased sodium, decreased potassium, decreased magnesium, and decreases in white blood cells, hemoglobin, neutrophils, platelets, and lymphocytes. ( 6.1)
RYBREVANT as a Single Agent
- The most common adverse reactions (≥ 20%) were rash, IRR, paronychia, musculoskeletal pain, dyspnea, nausea, fatigue, edema, stomatitis, cough, constipation, and vomiting. ( 6.1)
- The most common Grade 3 or 4 laboratory abnormalities (≥ 2%) were decreased lymphocytes, decreased albumin, decreased phosphate, decreased potassium, increased alkaline phosphatase, increased glucose, increased gamma-glutamyl transferase, and decreased sodium. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Biotech, Inc. at 1-800-JANSSEN (1-800-526-7736) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 First-Line Treatment of NSCLC with EGFRExon 20 Insertion Mutations
1.2 Previously Treated NSCLC with EGFRExon 20 Insertion Mutations
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
2.2 Patient Selection
2.3 Recommended Dosage of RYBREVANT for First-line Treatment of NSCLC with Exon 20 Insertion Mutations (RYBREVANT in Combination with Carboplatin and Pemetrexed)
2.4 Recommended Dosage of RYBREVANT for Patients with Previously Treated NSCLC with Exon 20 Insertion Mutations (RYBREVANT as a Single Agent)
2.5 Recommended Premedications
2.6 Dosage Modifications for Adverse Reactions
2.7 Preparation
2.8 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Infusion-Related Reactions
5.2 Interstitial Lung Disease/Pneumonitis
5.3 Dermatologic Adverse Reactions
5.4 Ocular Toxicity
5.5 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 First Line Treatment of NSCLC with Exon 20 Insertion Mutations-PAPILLON
14.2 Previously Treated NSCLC with Exon 20 Insertion Mutations-CHRYSALIS
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 First-Line Treatment of NSCLC with EGFRExon 20 Insertion Mutations
RYBREVANT is indicated in combination with carboplatin and pemetrexed for the first-line treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, as detected by an FDA-approved test [see Dosage and Administration (2.2)] .
1.2 Previously Treated NSCLC with EGFRExon 20 Insertion Mutations
RYBREVANT is indicated as a single agent for the treatment of adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, as detected by an FDA-approved test [see Dosage and Administration (2.2)] , whose disease has progressed on or after platinum-based chemotherapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
- Administer premedications before each RYBREVANT infusion as recommended [see Dosage and Administration (2.5)].
- Administer diluted RYBREVANT intravenously according to the infusion rates in Tables 7 and 8, with the initial dose as a split infusion on Week 1 on Day 1 and Day 2 [see Dosage and Administration (2.8)].
- Administer RYBREVANT via peripheral line for Week 1 Day 1 and 2 and Week 2 to reduce the risk of infusion-related reactions [see Dosage and Administration 2.8].
- When administering RYBREVANT in combination with carboplatin and pemetrexed, infuse pemetrexed first, carboplatin second, and RYBREVANT last [see Dosage and Administration 2.8].
2.2 Patient Selection
Select patients for treatment with RYBREVANT based on the presence of EGFR exon 20 insertion mutations in tumor or plasma specimens [see Clinical Studies (14)]. If no mutation is detected in a plasma specimen, test tumor tissue. Information on FDA-approved tests is available at: http://www.fda.gov/CompanionDiagnostics.
2.3 Recommended Dosage of RYBREVANT for First-line Treatment of NSCLC with Exon 20 Insertion Mutations (RYBREVANT in Combination with Carboplatin and Pemetrexed)
The recommended dosages of RYBREVANT, administered in combination with carboplatin and pemetrexed, based on baseline body weight are provided in Table 1.
Table 1: Recommended Dosage for RYBREVANT in Combination with Carboplatin and Pemetrexed Body weight at Baseline * Recommended Dose Dosing Schedule - *
- Dose adjustments not required for subsequent body weight changes.
Less than 80 kg 1400 mg Weekly (total of 4 doses) from Weeks 1 to 4 - Week 1 - split infusion on Day 1 and Day 2
- Weeks 2 to 4 - infusion on Day 1
- Weeks 5 and 6 – no dose
1750 mg Every 3 weeks starting at Week 7 onwards Greater than or equal to 80 kg 1750 mg Weekly (total of 4 doses) for Weeks 1 to 4 - Week 1 - split infusion on Day 1 and Day 2
- Weeks 2 to 4 - infusion on Day 1
- Weeks 5 and 6 – no dose
2100 mg Every 3 weeks starting at Week 7 onwards The recommended order of administration and regimen for RYBREVANT in combination with carboplatin and pemetrexed is provided in Table 2.
Table 2: Order of Administration and Regimen for RYBREVANT in Combination with Pemetrexed and Carboplatin RYBREVANT in Combination with Carboplatin and Pemetrexed Administer the regimen in the following order: pemetrexed first, carboplatin second and RYBREVANT last. Drug Dose Duration/Timing of Treatment Pemetrexed Pemetrexed 500 mg/m 2intravenously
Refer to the pemetrexed Full Prescribing Information for complete information.Every 3 weeks, continue until disease progression or unacceptable toxicity. Carboplatin Carboplatin AUC 5 intravenously
Refer to the carboplatin Full Prescribing Information for complete information.Every 3 weeks for up to 12 weeks. RYBREVANT RYBREVANT intravenously
See Table 1.Every 3 weeks, continue until disease progression or unacceptable toxicity. 2.4 Recommended Dosage of RYBREVANT for Patients with Previously Treated NSCLC with Exon 20 Insertion Mutations (RYBREVANT as a Single Agent)
The recommended dosages of RYBREVANT as a single agent, based on baseline body weight, are provided in Table 3.
Table 3: Recommended Dosage Schedule for RYBREVANT as a Single Agent Body weight at Baseline * Recommended Dose Dosing Schedule - *
- Dose adjustments not required for subsequent body weight changes.
Less than 80 kg 1050 mg Weekly (total of 5 doses) from Weeks 1 to 5 - Week 1 - split infusion on Day 1 and Day 2
- Weeks 2 to 5 - infusion on Day 1
- Week 6 – no dose
Every 2 weeks starting at Week 7 onwards Greater than or equal to 80 kg 1400 mg Weekly (total of 5 doses) from Weeks 1 to 5 - Week 1 - split infusion on Day 1 and Day 2
- Weeks 2 to 5 - infusion on Day 1
- Week 6 – no dose
Every 2 weeks starting at Week 7 onwards Administer RYBREVANT until disease progression or unacceptable toxicity.
2.5 Recommended Premedications
Prior to the initial infusion of RYBREVANT (Week 1, Day 1 and 2), administer premedication as described in Table 4 to reduce the risk of infusion-related reactions [see Warnings and Precautions (5.1)].
Glucocorticoid administration is required for Week 1, Day 1 and 2 dose only and upon re-initiation after prolonged dose interruptions, then as necessary for subsequent infusions (see Table 4). Administer both antihistamine and antipyretic prior to all infusions.
Table 4: Premedications Medication Dose Route of Administration Dosing Window Prior to RYBREVANT Administration Antihistamine * Diphenhydramine (25 to 50 mg) or equivalent Intravenous 15 to 30 minutes Oral 30 to 60 minutes Antipyretic * Acetaminophen (650 to 1,000 mg) Intravenous 15 to 30 minutes Oral 30 to 60 minutes Glucocorticoid † Dexamethasone (20 mg) or equivalent Intravenous 45 to 60 minutes Glucocorticoid ‡ Dexamethasone (10 mg) or equivalent Intravenous 45 to 60 minutes 2.6 Dosage Modifications for Adverse Reactions
The recommended dose reductions for adverse reactions for RYBREVANT are listed in Table 5.
Table 5: Dose Reductions for Adverse Reactions for RYBREVANT Dose * 1 stDose Reduction 2 ndDose Reduction 3 rdDose Reduction - *
- Dose at which the adverse reaction occurred
1050 mg 700 mg 350 mg Discontinue RYBREVANT 1400 mg 1050 mg 700 mg 1750 mg 1400 mg 1050 mg 2100 mg 1750 mg 1400 mg The recommended dosage modifications and management for adverse reactions for RYBREVANT are provided in Table 6.
Table 6: Recommended Dosage Modifications and Management for Adverse Reactions for RYBREVANT Adverse Reaction Severity Dosage Modifications Infusion-related reactions (IRR) [see Warnings and Precautions (5.1)] Grade 1 to 2 - Interrupt RYBREVANT infusion if IRR is suspected and monitor patient until reaction symptoms resolve.
- Resume the infusion at 50% of the infusion rate at which the reaction occurred.
- If there are no additional symptoms after 30 minutes, the infusion rate may be escalated (see Tables 7and 8).
- Include corticosteroid with premedications for subsequent dose (see Table 4).
Grade 3 - Interrupt RYBREVANT infusion and administer supportive care medications. Continuously monitor patient until reaction symptoms resolve.
- Resume the infusion at 50% of the infusion rate at which the reaction occurred.
- If there are no additional symptoms after 30 minutes, the infusion rate may be escalated (see Tables 7and 8).
- Include corticosteroid with premedications for subsequent dose (see Table 4). For recurrent Grade 3, permanently discontinue RYBREVANT.
Grade 4 - Permanently discontinue RYBREVANT.
Interstitial Lung Disease (ILD)/pneumonitis [see Warnings and Precautions (5.2)] Any Grade - Withhold RYBREVANT if ILD/pneumonitis is suspected.
- Permanently discontinue RYBREVANT if ILD/pneumonitis is confirmed.
Dermatologic Adverse Reactions (including dermatitis acneiform, pruritus, dry skin) [see Warnings and Precautions (5.3)] Grade 1 - Initiate supportive care management.
- Reassess after 2 weeks.
Grade 2 - Initiate supportive care management.
- Reassess after 2 weeks; if rash does not improve, consider dose reduction.
Grade 3 - Withhold RYBREVANT and initiate supportive care management.
- Upon recovery to ≤ Grade 2, resume RYBREVANT at reduced dose.
- If no improvement within 2 weeks, permanently discontinue treatment.
Grade 4 - Permanently discontinue RYBREVANT.
Severe bullous, blistering or exfoliating skin conditions (including toxic epidermal necrolysis (TEN) - Permanently discontinue RYBREVANT.
Other Adverse Reactions [see Adverse Reactions (6.1)] Grade 3 - Withhold RYBREVANT until recovery to ≤ Grade 1 or baseline.
- Resume at the same dose if recovery occurs within 1 week.
- Resume at reduced dose if recovery occurs after 1 week but within 4 weeks.
- Permanently discontinue if recovery does not occur within 4 weeks.
Grade 4 - Withhold RYBREVANT until recovery to ≤Grade 1 or baseline.
- Resume at reduced dose if recovery occurs within 4 weeks.
- Permanently discontinue if recovery does not occur within 4 weeks.
- Permanently discontinue for recurrent Grade 4 reactions.
Recommended Dosage Modifications for Adverse Reactions for RYBREVANT in Combination with Carboplatin and Pemetrexed
When administering RYBREVANT in combination with carboplatin and pemetrexed, modify the dosage of one or more drugs. Withhold or discontinue RYBREVANT as shown in Table 6. Refer to prescribing information for carboplatin and pemetrexed for additional dosage modification information.
2.7 Preparation
Dilute and prepare RYBREVANT for intravenous infusion before administration.
- Check that the RYBREVANT solution is colorless to pale yellow. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if discoloration or visible particles are present.
- Determine the dose required of RYBREVANT needed based on patient's baseline weight [see Dosage and Administration (2.3)] . Each vial of RYBREVANT contains 350 mg of amivantamab-vmjw.
- Withdraw and then discard a volume of either 5% dextrose solution or 0.9% sodium chloride solution from the 250 mL infusion bag equal to the volume of RYBREVANT to be added (i.e., discard 7 mL diluent from the infusion bag for each RYBREVANT vial). Only use infusion bags made of polyvinylchloride (PVC), polypropylene (PP), polyethylene (PE), or polyolefin blend (PP+PE).
- Withdraw 7 mL of RYBREVANT from each vial and add it to the infusion bag. The final volume in the infusion bag should be 250 mL. Discard any unused portion left in the vial.
- Gently invert the bag to mix the solution. Do not shake.
- Diluted solutions should be administered within 10 hours (including infusion time) at room temperature 59°F to 77°F (15°C to 25°C).
2.8 Administration
- Administer the diluted RYBREVANT solution [see Dosage and Administration (2.7)] by intravenous infusion using an infusion set fitted with a flow regulator and with an in-line, sterile, non-pyrogenic, low protein-binding polyethersulfone (PES) filter (pore size 0.2 micrometer).
- Administration sets must be made of either polyurethane (PU), polybutadiene (PBD), PVC, PP, or PE.
- The administration set with filter, mustbe primed with either 5% dextrose solution or 0.9% sodium chloride solution prior to the initiation of each RYBREVANT infusion.
- Do not infuse RYBREVANT concomitantly in the same intravenous line with other agents.
RYBREVANT in Combination with Carboplatin and Pemetrexed
- Administer RYBREVANT in combination with carboplatin and pemetrexed infusions every 3 weeks intravenously according to the infusion rates in Table 7.
- Administer RYBREVANT via a peripheral line on Week 1 and Week 2 given the high incidence of infusion-related reactions during initial treatment [see Warnings and Precautions (5.1)].
- RYBREVANT may be administered via central line for subsequent weeks.
- For the initial infusion, prepare RYBREVANT as close to administration time as possible to allow for the possibility of extended infusion time in the event of an infusion related reaction.
- Administer the pemetrexed infusion first, carboplatin infusion second, and the RYBREVANT infusion last.
Table 7: Infusion Rates of RYBREVANT for First-line Treatment of NSCLC with Exon 20 Insertion Mutations (RYBREVANT in Combination with Carboplatin and Pemetrexed) - *
- In the absence of infusion-related reactions, increase the initial infusion rate to the subsequent infusion rate after 2 hours based on patient tolerance. Total infusion time approximately 4–6 hours for day 1 and 6–8 hours for day 2. Subsequent infusion time is approximately 2 hours.
Body Weight Less Than 80 kg Week Dose
(per 250 mL bag)Initial Infusion Rate
(mL/hr)Subsequent Infusion Rate*
(mL/hr)Week 1 (split dose infusion) Week 1 Day 1 350 mg 50 75 Week 1 Day 2 1050 mg 33 50 Week 2 1400 mg 65 Week 3 1400 mg 85 Week 4 1400 mg 125 Weeks 5 and 6 No dose Week 7 and every 3 weeks thereafter 1750 mg 125 Body Weight Greater Than or Equal to 80 kg Week Dose
(per 250 mL bag)Initial Infusion Rate
(mL/hr)Subsequent Infusion Rate
(mL/hr)Week 1 (split dose infusion) Week 1 Day 1 350 mg 50 75 Week 1 Day 2 1400 mg 25 50 Week 2 1750 mg 65 Week 3 1750 mg 85 Week 4 1750 mg 125 Week 5 and 6 No dose Week 7 and every 3 weeks thereafter 2100 mg 125 RYBREVANT as a Single Agent
- Administer RYBREVANT as a single agent infusion every 2 weeks intravenously according to the infusion rates in Table 8.
- Administer RYBREVANT via a peripheral line on Week 1 and Week 2, given the high incidence of infusion-related reactions during initial treatment [see Warnings and Precautions (5.1)].
- RYBREVANT may be administered via central line for subsequent weeks.
- For the initial infusion, prepare RYBREVANT as close to administration time as possible to allow for the possibility of extended infusion time in the event of an infusion related reaction.
Table 8: Infusion Rates of RYBREVANT for Patients with Previously Treated NSCLC with Exon 20 Insertion Mutations (RYBREVANT as Single Agent) - *
- In the absence of infusion-related reactions, increase the initial infusion rate to the subsequent infusion rate after 2 hours based on patient tolerance. Total infusion time approximately 4–6 hours for day 1 and 6–8 hours for day 2. Subsequent infusion time is approximately 2 hours.
Body Weight Less Than 80 kg Week Dose
(per 250 mL bag)Initial Infusion Rate
(mL/hr)Subsequent Infusion Rate*
(mL/hr)Week 1 (split dose infusion) Week 1 Day 1 350 mg 50 75 Week 1 Day 2 700 mg 50 75 Week 2 1050 mg 85 Week 3 1050 mg 125 Week 4 1050 mg 125 Week 5 1050 mg 125 Week 6 No dose Week 7 and every 2 weeks thereafter 1050 mg 125 Body Weight Greater Than or Equal to 80 kg Week Dose
(per 250 mL bag)Initial Infusion Rate
(mL/hr)Subsequent Infusion Rate *
(mL/hr)Week 1 (split dose infusion) Week 1 Day 1 350 mg 50 75 Week 1 Day 2 1050 mg 35 50 Week 2 1400 mg 65 Week 3 1400 mg 85 Week 4 1400 mg 125 Week 5 1400 mg 125 Week 6 No dose Week 7 and every 2 weeks thereafter 1400 mg 125 - 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Infusion-Related Reactions
RYBREVANT can cause infusion-related reactions (IRR); signs and symptoms of IRR include dyspnea, flushing, fever, chills, nausea, chest discomfort, hypotension and vomiting.
RYBREVANT with Carboplatin and Pemetrexed
RYBREVANT in combination with carboplatin and pemetrexed can cause infusion-related reactions. Based on the safety population [see Adverse Reactions (6.1)] , infusion-related reactions occurred in 42% of patients treated with RYBREVANT in combination with carboplatin and pemetrexed, including Grade 3 (1.3%) adverse reactions. The incidence of infusion modifications due to IRR was 40%, and 0.7% of patients permanently discontinued RYBREVANT.
RYBREVANT as a Single Agent
Based on the safety population [see Adverse Reactions (6.1)], IRR occurred in 66% of patients treated with RYBREVANT as a single agent. Among patients receiving treatment on Week 1 Day 1, 65% experienced an IRR, while the incidence of IRR was 3.4%with the Day 2 infusion, 0.4% with the Week 2 infusion, and cumulatively 1.1% with subsequent infusions. Of the reported IRRs, 97% were Grade 1–2, 2.2% were Grade 3, and 0.4% were Grade 4. The median time to onset was 1 hour (range 0.1 to 18 hours) after start of infusion. The incidence of infusion modifications due to IRR was 62%, and 1.3% of patients permanently discontinued RYBREVANT due to IRR.
Premedicate with antihistamines, antipyretics, and glucocorticoids and infuse RYBREVANT as recommended [see Dosage and Administration (2.4)] . Administer RYBREVANT via a peripheral line on Week 1 and Week 2 [see Dosage and Administration (2.7)] .
Monitor patients for any signs and symptoms of infusion reactions during RYBREVANT infusion in a setting where cardiopulmonary resuscitation medication and equipment are available. Interrupt infusion if IRR is suspected. Reduce the infusion rate or permanently discontinue RYBREVANT based on severity [see Dosage and Administration (2.5)].
5.2 Interstitial Lung Disease/Pneumonitis
RYBREVANT can cause interstitial lung disease (ILD)/pneumonitis.
RYBREVANT with Carboplatin and Pemetrexed
Based on the safety population [see Adverse Reactions (6.1)], Grade 3 ILD/pneumonitis occurred in 2.6% of patients treated with RYBREVANT in combination with carboplatin and pemetrexed, all patients required permanent discontinuation.
RYBREVANT as a Single Agent
Based on the safety population [see Adverse Reactions (6.1)] , ILD/pneumonitis occurred in 3.3% of patients treated with RYBREVANT as a single agent, with 0.7 % of patients experiencing Grade 3 ILD/pneumonitis. Three patients (1%) discontinued RYBREVANT due to ILD/pneumonitis.
Monitor patients for new or worsening symptoms indicative of ILD/pneumonitis (e.g., dyspnea, cough, fever). Immediately withhold RYBREVANT in patients with suspected ILD/pneumonitis and permanently discontinue if ILD/pneumonitis is confirmed [see Dosage and Administration (2.5)] .
5.3 Dermatologic Adverse Reactions
RYBREVANT can cause rash (including dermatitis acneiform), pruritus and dry skin.
RYBREVANT with Carboplatin and Pemetrexed
RYBREVANT in combination with carboplatin and pemetrexed can cause dermatologic adverse reactions. Based on the safety population [see Adverse Reactions (6.1)] , rash occurred in 89% of patients treated with RYBREVANT in combination with carboplatin and pemetrexed, including Grade 3 (19%) adverse reactions. Rash leading to dose reductions occurred in 19% of patients, and 2% permanently discontinued RYBREVANT and 1.3% discontinued pemetrexed.
RYBREVANT as a Single Agent
Based on the safety population [see Adverse Reactions (6.1)] , rash occurred in 74% of patients treated with RYBREVANT as a single agent, including Grade 3 rash in 3.3% of patients. The median time to onset of rash was 14 days (range: 1 to 276 days). Rash leading to dose reduction occurred in 5% of patients, and RYBREVANT was permanently discontinued due to rash in 0.7% of patients [see Adverse Reactions (6.1)].
Toxic epidermal necrolysis (TEN) occurred in one patient (0.3%) treated with RYBREVANT as a single agent.
Instruct patients to limit sun exposure during and for 2 months after treatment with RYBREVANT. Advise patients to wear protective clothing and use broad-spectrum UVA/UVB sunscreen. Alcohol-free emollient cream is recommended for dry skin.
If skin reactions develop, start topical corticosteroids and topical and/or oral antibiotics. For Grade 3 reactions, add oral steroids and consider dermatologic consultation. Promptly refer patients presenting with severe rash, atypical appearance or distribution, or lack of improvement within 2 weeks to a dermatologist. Withhold, dose reduce or permanently discontinue RYBREVANT based on severity [see Dosage and Administration (2.6)] .
5.4 Ocular Toxicity
RYBREVANT can cause ocular toxicity including keratitis, dry eye symptoms, conjunctival redness, blurred vision, visual impairment, ocular itching, and uveitis.
RYBREVANT with Carboplatin and Pemetrexed
Based on the safety population [see Adverse Reactions (6.1)] RYBREVANT in combination with carboplatin and pemetrexed can cause ocular toxicity including blepharitis, dry eye, conjunctival redness, blurred vision, and eye pruritus. All events were Grade 1–2.
RYBREVANT as a Single Agent
Based on the safety population [see Adverse Reactions (6.1)], keratitis occurred in 0.7% and uveitis occurred in 0.3% of patients treated with RYBREVANT. All events were Grade 1–2. Promptly refer patients presenting with eye symptoms to an ophthalmologist. Withhold, dose reduce or permanently discontinue RYBREVANT based on severity [see Dosage and Administration (2.6)] .
5.5 Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal models, RYBREVANT can cause fetal harm when administered to a pregnant woman. Administration of other EGFR inhibitor molecules to pregnant animals has resulted in an increased incidence of impairment of embryo-fetal development, embryo lethality, and abortion. Advise females of reproductive potential of the potential risk to the fetus. Advise female patients of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of RYBREVANT. [see Use in Specific Populations (8.1, 8.3)] .
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere in the labeling:
- Infusion-Related Reactions [see Warnings and Precautions (5.1)]
- Interstitial Lung Disease/Pneumonitis [see Warnings and Precautions (5.2)]
- Dermatologic Adverse Reactions [see Warnings and Precautions (5.3)]
- Ocular Toxicity [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in the WARNINGS AND PRECAUTIONS reflect exposure to RYBREVANT in combination with carboplatin and pemetrexed in the PAPILLON study in 151 patients with locally advanced or metastatic NSCLC. Patients received RYBREVANT intravenously at 1400 mg (for patients < 80 kg) or 1750 mg (for patients ≥ 80 kg) once weekly through 4 weeks, then every 3 weeks with a dose of 1750 mg (for patients < 80 kg) or 2100 mg (for patients ≥ 80 kg) starting at Week 7 until disease progression or unacceptable toxicity, in combination with carboplatin at area under the curve AUC 5 once every 3 weeks, for up to 12 weeks, and pemetrexed at 500 mg/m2 once every 3 weeks until disease progression or unacceptable toxicity. Among 151 patients who received RYBREVANT in combination with carboplatin and pemetrexed, 76% were exposed for 6 months or longer and 38% were exposed for greater than one year. In the safety population, the most common (≥ 20%) adverse reactions were rash, nail toxicity, stomatitis, infusion-related reaction, fatigue, edema, constipation, decreased appetite, nausea, COVID-19, diarrhea, and vomiting. The most common Grade 3 to 4 laboratory abnormalities (≥ 2%) were increased alanine aminotransferase decreased albumin, decreased potassium and decreased magnesium.
The data in the WARNINGS AND PRECAUTIONS also reflect exposure to RYBREVANT as a single agent in the CHRYSALIS study in 302 patients with locally advanced or metastatic NSCLC. Patients received RYBREVANT at 1050 mg (for patient baseline body weight < 80 kg) or 1400 mg (for patient baseline body weight ≥80 kg) once weekly for 4 weeks, then every 2 weeks thereafter until disease progression or unacceptable toxicity. Among 302 patients who received RYBREVANT as a single agent, 36% were exposed for 6 months or longer and 12% were exposed for greater than one year. In the safety population, the most common (≥ 20%) adverse reactions were rash, infusion-related reaction, paronychia, musculoskeletal pain, dyspnea, nausea, edema, cough, fatigue, stomatitis, constipation, vomiting and pruritus. The most common Grade 3 to 4 laboratory abnormalities (≥ 2%) were increased gamma glutamyl transference, decreased sodium, decreased potassium and increased alkaline phosphatase.
First-line Treatment of Non-Small Cell Lung Cancer (NSCLC) with Exon 20 Insertion Mutations
The data described below reflect exposure to RYBREVANT in combination with carboplatin and pemetrexed at the recommended dosage in the PAPILLON trial [see Clinical Studies (14.1)] in 151 patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations. Among patients who received RYBREVANT in combination with carboplatin and pemetrexed the median exposure was 9.7 months (range: 0.0 to 26.9 months). In patients that received carboplatin and pemetrexed alone, the median exposure was 6.7 months (range 0.0 to 25.3).
The median age was 61 years (range: 27 to 86 years); 56% were female; 64% were Asian, 32% were White, 1.3% were Black or African American, race was not reported in 1.3% of patients; 89% were not Hispanic or Latino; 86% had baseline body weight <80 kg.
Serious adverse reactions occurred in 37% of patients who received RYBREVANT in combination with carboplatin and pemetrexed. Serious adverse reactions in ≥ 2% of patients included rash, pneumonia, interstitial lung disease (ILD), pulmonary embolism, vomiting and COVID-19. Fatal adverse reactions occurred in 7 patients (4.6%) due to pneumonia, cerebrovascular accident, cardio-respiratory arrest, COVID-19, sepsis and death not otherwise specified.
Permanent discontinuation of RYBREVANT due to an adverse reaction occurred in 11% of patients. Adverse reactions resulting in permanent discontinuation of RYBREVANT in ≥1% of patients were rash and ILD.
Dose interruptions of RYBREVANT due to an adverse reaction occurred in 64% of patients. Infusion-related reactions (IRR) requiring infusion interruptions occurred in 38% of patients. Adverse reactions requiring dose interruption in ≥5% of patients included rash and nail toxicity.
Dose reductions of RYBREVANT due to an adverse reaction occurred in 36% of patients. Adverse reactions requiring dose reductions in ≥ 5% of patients included rash, and nail toxicity.
The most common adverse reactions (≥ 20%) were rash, nail toxicity, stomatitis, infusion-related reaction, fatigue, edema, constipation, decreased appetite, nausea, COVID-19, diarrhea, and vomiting. The most common Grade 3 to 4 laboratory abnormalities (≥ 2%) were decreased albumin, increased alanine aminotransferase, increased gamma-glutamyl transferase, decreased sodium, decreased potassium, decreased magnesium, and decreases in white blood cells, hemoglobin, neutrophils, platelets, and lymphocytes.
Table 9 summarizes the adverse reactions in PAPILLON.
Table 9: Adverse Reactions (≥10%) in Patients with Metastatic NSCLC with Exon 20 Insertion Mutations Who Received RYBREVANT in Combination with Carboplatin and Pemetrexed in PAPILLON Adverse Reaction * RYBREVANT in Combination with Carboplatin and Pemetrexed
(n=151)Carboplatin and Pemetrexed
(n=155)All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)Skin and subcutaneous tissue disorders Rash † 90 19 19 0 Nail toxicity † 62 7 3 0 Dry skin † 17 0 6 0 Gastrointestinal disorders Stomatitis † 43 4 11 0 Constipation 40 0 30 0.7 Nausea 36 0.7 42 0 Vomiting 21 3.3 19 0.7 Diarrhea 21 3 13 1.3 Hemorrhoids 12 1 1.3 0 Abdominal pain † 11 0.7 8 0 General disorders and administration site conditions Infusion related reaction 42 1.3 1.3 0 Fatigue † 42 6 45 3.9 Edema † 40 1.3 19 0 Pyrexia † 17 0 6 0 Metabolism and nutrition disorders Decreased appetite 36 2.6 28 1.3 Infections and Infestations COVID-19 24 2 14 0.6 Pneumonia † 13 5 6 1.9 Vascular Disorders Hemorrhage † 18 0.7 11 1.9 Respiratory, thoracic and mediastinal disorders Cough † 17 0 16 0 Dyspnea † 11 1.3 16 3.2 Investigations Weight decreased 14 0.7 8 0 Nervous System Disorders Dizziness † 11 0 12 0 Psychiatric Disorders Insomnia 11 0 13 0 Clinically relevant adverse reactions in <10% of patients who received RYBREVANT in combination with carboplatin and pemetrexed included pulmonary embolism, deep vein thrombosis, skin ulcer, conjunctivitis and interstitial lung disease (ILD)/pneumonitis.
Table 10 summarizes the laboratory abnormalities in PAPILLON.
Table 10: Select Laboratory Abnormalities (≥ 20%) That Worsened from Baseline in Patients With Metastatic NSCLC with EGFR Exon 20 Insertion Mutations Who Received RYBREVANT in Combination with Carboplatin and Pemetrexed in PAPILLON Laboratory Abnormality * RYBREVANT in Combination with Carboplatin and Pemetrexed † Carboplatin in Combination with Pemetrexed ‡ All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- *
- Adverse reactions were graded using CTCAE version 5.0
- †
- The denominator used to calculate the rate varied from 113 to 150 based on the number of patients with a baseline value and at least one post-treatment value.
- ‡
- The denominator used to calculate the rate varied from 119 to 154 based on the number of patients with a baseline value and at least one post-treatment value.
Hematology Decreased white blood cells 89 17 76 10 Decreased hemoglobin 79 11 85 13 Decreased neutrophils 76 36 61 23 Decreased platelets 70 10 54 12 Decreased lymphocytes 61 11 49 13 Chemistry Decreased albumin 87 7 34 1 Increased aspartate aminotransferase 60 1 61 1 Increased alanine aminotransferase 57 4 54 1 Decreased sodium 55 7 39 4 Increased alkaline phosphatase 51 1 28 0 Decreased potassium 44 11 17 1 Decreased magnesium 39 2 30 1 Increased gamma-glutamyl transferase 38 4 43 4 Decreased calcium (corrected) 27 1 18 1 Previously Treated NSCLC Exon 20 Insertion Mutations
The data described below reflect exposure to RYBREVANT at the recommended dosage in 129 patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations in the CHRYSALIS trial [see Clinical Studies (14.2)], whose disease had progressed on or after platinum-based chemotherapy. Among patients who received RYBREVANT, 44% were exposed for 6 months or longer and 12% were exposed for greater than one year.
The median age was 62 years (range: 36 to 84 years); 61% were female; 55% were Asian, 35% were White, and 2.3% were Black; and 82% had baseline body weight <80 kg.
Serious adverse reactions occurred in 30% of patients who received RYBREVANT. Serious adverse reactions in ≥ 2% of patients included pulmonary embolism, pneumonitis/ILD, dyspnea, musculoskeletal pain, pneumonia, and muscular weakness. Fatal adverse reactions occurred in 2 patients (1.5%) due to pneumonia and 1 patient (0.8%) due to sudden death.
Permanent discontinuation of RYBREVANT due to an adverse reaction occurred in 11% of patients. Adverse reactions resulting in permanent discontinuation of RYBREVANT in ≥1% of patients were pneumonia, IRR, pneumonitis/ILD, dyspnea, pleural effusion, and rash.
Dose interruptions of RYBREVANT due to an adverse reaction occurred in 78% of patients. Infusion-related reactions (IRR) requiring infusion interruptions occurred in 59% of patients. Adverse reactions requiring dose interruption in ≥5% of patients included dyspnea, nausea, rash, vomiting, fatigue, and diarrhea.
Dose reductions of RYBREVANT due to an adverse reaction occurred in 15% of patients. Adverse reactions requiring dose reductions in ≥ 2% of patients included rash and paronychia.
The most common adverse reactions (≥ 20%) were rash, IRR, paronychia, musculoskeletal pain, dyspnea, nausea, fatigue, edema, stomatitis, cough, constipation, and vomiting. The most common Grade 3 to 4 laboratory abnormalities (≥ 2%) were decreased lymphocytes, decreased albumin, decreased phosphate, decreased potassium, increased glucose, increased alkaline phosphatase, increased gamma-glutamyl transferase, and decreased sodium.
Table 11 summarizes the adverse reactions in CHRYSALIS.
Table 11: Adverse Reactions (≥ 10%) in Patients with NSCLC with Exon 20 Insertion Mutations Whose Disease Has Progressed on or after Platinum-based Chemotherapy and Received RYBREVANT in CHRYSALIS Adverse Reactions RYBREVANT *
(N=129)All Grades (%) Grades 3 or 4 (%) Skin and subcutaneous tissue disorders Rash † 84 3.9 Pruritus 18 0 Dry skin 14 0 General disorders and administration site conditions Infusion related reaction 64 3.1 Fatigue † 33 2.3 Edema † 27 0.8 Pyrexia 13 0 Infections and infestations Paronychia 50 3.1 Pneumonia † 10 0.8 Musculoskeletal and connective tissue disorders Musculoskeletal pain † 47 0 Respiratory, thoracic and mediastinal disorders Dyspnea † 37 2.3 Cough † 25 0 Gastrointestinal disorders Nausea 36 0 Stomatitis † 26 0.8 Constipation 23 0 Vomiting 22 0 Diarrhea 16 3.1 Abdominal Pain † 11 0.8 Vascular disorders Hemorrhage † 19 0 Metabolism and nutrition disorders Decreased appetite 15 0 Nervous system disorders Peripheral neuropathy † 13 0 Dizziness 12 0.8 Headache † 10 0.8 Clinically relevant adverse reactions in <10% of patients who received RYBREVANT included ocular toxicity, ILD/pneumonitis, and toxic epidermal necrolysis (TEN).
Table 12 summarizes the laboratory abnormalities in CHRYSALIS.
Table 12: Select Laboratory Abnormalities (≥ 20%) That Worsened from Baseline in Patients With Metastatic NSCLC with EGFR Exon 20 Insertion Mutations Whose Disease Has Progressed on or After Platinum-based Chemotherapy and Who Received RYBREVANT in CHRYSALIS Laboratory Abnormality RYBREVANT *
(N=129)All Grades
(%)Grades 3 or 4
(%)- *
- The denominator used to calculate the rate was 126 based on the number of patients with a baseline value and at least one post-treatment value.
Chemistry Decreased albumin 79 8 Increased glucose 56 4 Increased alkaline phosphatase 53 4.8 Increased creatinine 46 0 Increased alanine aminotransferase 38 1.6 Decreased phosphate 33 8 Increased aspartate aminotransferase 33 0 Decreased magnesium 27 0 Increased gamma-glutamyl transferase 27 4 Decreased sodium 27 4 Decreased potassium 26 6 Hematology Decreased lymphocytes 36 8 -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on the mechanism of action and findings in animal models, RYBREVANT can cause fetal harm when administered to a pregnant woman. There are no available data on the use of RYBREVANT in pregnant women or animal data to assess the risk of RYBREVANT in pregnancy. Disruption or depletion of EGFR in animal models resulted in impairment of embryo-fetal development including effects on placental, lung, cardiac, skin, and neural development. The absence of EGFR or MET signaling has resulted in embryo lethality, malformations, and post-natal death in animals ( see Data) .Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
No animal studies have been conducted to evaluate the effects of amivantamab-vmjw on reproduction and fetal development; however, based on its mechanism of action, RYBREVANT can cause fetal harm or developmental anomalies. In mice, EGFR is critically important in reproductive and developmental processes including blastocyst implantation, placental development, and embryo-fetal/postnatal survival and development. Reduction or elimination of embryo-fetal or maternal EGFR signaling can prevent implantation, can cause embryo-fetal loss during various stages of gestation (through effects on placental development) and can cause developmental anomalies and early death in surviving fetuses. Adverse developmental outcomes were observed in multiple organs in embryos/neonates of mice with disrupted EGFR signaling. Similarly, knock out of MET or its ligand HGF was embryonic lethal due to severe defects in placental development, and fetuses displayed defects in muscle development in multiple organs. Human IgG1 is known to cross the placenta; therefore, amivantamab-vmjw has the potential to be transmitted from the mother to the developing fetus.
8.2 Lactation
Risk Summary
There are no data on the presence of amivantamab-vmjw in human milk, the effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions from RYBREVANT in breast-fed children, advise women not to breast-feed during treatment with RYBREVANT and for 3 months after the last dose.
8.3 Females and Males of Reproductive Potential
RYBREVANT can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and efficacy of RYBREVANT have not been established in pediatric patients.
8.5 Geriatric Use
Of the 151 patients with locally advanced or metastatic NSCLC treated with RYBREVANT in combination with carboplatin and pemetrexed in the PAPILLON study, 37% were ≥65 years of age and 8% were ≥75 years of age.
Of the 302 patients with locally advanced or metastatic NSCLC treated with RYBREVANT as a single agent in the CHRYSALIS study, 39% were ≥65 years of age and 11% were ≥75 years of age.
No clinically important differences in safety or efficacy were observed between patients who were ≥65 years of age and younger patients.
-
11 DESCRIPTION
Amivantamab-vmjw is a low-fucose human immunoglobulin G1-based bispecific antibody directed against the EGF and MET receptors, produced by mammalian cell line (Chinese Hamster Ovary [CHO]) using recombinant DNA technology that has a molecular weight of approximately 148 kDa. RYBREVANT ®(amivantamab-vmjw) injection for intravenous infusion is a sterile, preservative-free, colorless to pale yellow solution in single-dose vials. The pH is 5.7.
Each RYBREVANT vial contains 350 mg (50 mg/mL) amivantamab-vmjw, EDTA disodium salt dihydrate (0.14 mg), L-histidine (2.3 mg), L-histidine hydrochloride monohydrate (8.6 mg), L-methionine (7 mg), polysorbate 80 (4.2 mg), sucrose (595 mg), and water for injection, USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Amivantamab-vmjw is a bispecific antibody that binds to the extracellular domains of EGFR and MET.
In in vitroand in vivostudies amivantamab-vmjw was able to disrupt EGFR and MET signaling functions through blocking ligand binding and, in exon 20 insertion mutation models, degradation of EGFR and MET. The presence of EGFR and MET on the surface of tumor cells also allows for targeting of these cells for destruction by immune effector cells, such as natural killer cells and macrophages, through antibody-dependent cellular cytotoxicity (ADCC) and trogocytosis mechanisms, respectively.
12.2 Pharmacodynamics
The exposure-response relationship and time-course of pharmacodynamic response of amivantamab-vmjw have not been fully characterized in patients with NSCLC with EGFR exon 20 insertion mutations.
12.3 Pharmacokinetics
Based on RYBREVANT single agent data, amivantamab-vmjw exposures increased proportionally over a dosage range from 350 to 1750 mg. Based on the population pharmacokinetics of RYBREVANT, steady-state concentrations of RYBREVANT were reached by week 13 for both the 3-week and 2-week dosing regimen and the systemic accumulation was 1.9-fold.
Elimination
The geometric mean (% CV) linear clearance (CL) and terminal half-life is 0.266 L/day (30.4%) and 13.7 days (31.9%), respectively.
Specific Populations
No clinically meaningful differences in the pharmacokinetics of amivantamab-vmjw were observed based on age (range: 27–87 years), sex, race, creatinine clearance (CLcr 29 to 301 mL/min), or mild hepatic impairment [(total bilirubin ≤ ULN and AST > ULN) or (ULN < total bilirubin ≤ 1.5 times ULN)]. The pharmacokinetics of amivantamab-vmjw have not been studied in patients with severe renal impairment (CLcr 15 to 29 mL/min) or patients with moderate (total bilirubin 1.5 to 3 times ULN) to severe (total bilirubin > 3 times ULN) hepatic impairment.
Body Weight
Increases in body weight increased the volume of distribution and clearance of amivantamab-vmjw. Amivantamab-vmjw exposures are 30–40% lower in patients who weighed ≥ 80 kg compared to patients with body weight < 80 kg at the same dose. Exposures of amivantamab-vmjw were comparable between patients who weighed < 80 kg and received 1050 mg dose and patients who weighed ≥ 80 kg and received 1400 mg dose.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies (ADA) in the studies described below with the incidence of anti-drug antibodies in other studies, including those of amivantamab-vmjw or amivantamab products.
In patients with NSCLC who received RYBREVANT as a single agent or as part of a combination therapy, 3 of the 663 (0.5%) patients who were treated with RYBREVANT and evaluable for the presence of anti-drug antibodies (ADA) tested positive for treatment-emergent anti-amivantamab-vmjw antibodies. Given the low incidence of detectable antibody-drug antibodies, the effect of ADA on the efficacy of RYBREVANT remains unknown.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed to assess the potential of amivantamab-vmjw for carcinogenicity or genotoxicity. Fertility studies have not been performed to evaluate the potential effects of amivantamab-vmjw. In 6-week and 3-month repeat-dose toxicology studies in monkeys, there were no notable effects in the male and female reproductive organs.
-
14 CLINICAL STUDIES
14.1 First Line Treatment of NSCLC with Exon 20 Insertion Mutations-PAPILLON
The efficacy of RYBREVANT was evaluated in PAPILLON (NCT04538664), in a randomized, open-label, multicenter study. Eligible patients were required to have previously untreated locally advanced or metastatic NSCLC with EGFR Exon 20 insertion mutations measurable disease per RECIST v1.1, Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≤1, and adequate organ and bone marrow function. Patients with brain metastases at screening were eligible for participation once they were definitively treated, clinically stable, asymptomatic, and off corticosteroid treatment for at least 2 weeks prior to randomization. Patients with a medical history of interstitial lung disease or active ILD were excluded from the clinical study.
A total of 308 patients were randomized 1:1 to receive RYBREVANT in combination with carboplatin and pemetrexed (n=153) or carboplatin and pemetrexed (n=155). Patients received RYBREVANT intravenously at 1400 mg (for patients < 80 kg) or 1750 mg (for patients ≥ 80 kg) once weekly through 4 weeks, then every 3 weeks with a dose of 1750 mg (for patients < 80 kg) or 2100 mg (for patients ≥ 80 kg) starting at Week 7 until disease progression or unacceptable toxicity. Carboplatin was administered intravenously at area under the concentration-time curve 5 mg/mL per minute (AUC 5) once every 3 weeks, for up to 12 weeks. Pemetrexed was administered intravenously at 500 mg/m 2on once every 3 weeks until disease progression or unacceptable toxicity. Patients were stratified by Eastern Cooperative Oncology Group (ECOG) performance status (0 or 1) and prior brain metastases (yes or no).
The primary efficacy outcome measure was progression-free survival (PFS) as assessed by blinded independent central review (BICR). Additional efficacy outcome measures included overall response rate (ORR), duration of response (DOR) and overall survival (OS). Cross-over to single agent RYBREVANT was permitted for patients who had confirmed disease progression on carboplatin and pemetrexed.
The median age was 62 (range: 27 to 92) years, with 40% of the patients ≥ 65 years of age; 58% were female; 61% were Asian and 36% were White, 0.7% were Black or African American and race was not reported in 2.3% of patients; 93% were not Hispanic or Latino. Baseline ECOG performance status was 0 (35%) or 1 (65%); 58% were never smokers; 23% had history of brain metastasis and 84% had Stage IV cancer at initial diagnosis.
PAPILLON demonstrated a statistically significant improvement in progression free survival for patients randomized to RYBREVANT in combination with carboplatin and pemetrexed compared with carboplatin and pemetrexed.
Efficacy results are summarized in Table 13 and Figure 1.
Table 13: Efficacy Results in PAPILLON RYBREVANT+ carboplatin+ pemetrexed
(N=153)carboplatin+ pemetrexed
(N=155)CI = confidence interval Progression-Free Survival (PFS) Number of events (%) 84 (55) 132 (85) Median, months (95% CI) 11.4 (9.8, 13.7) 6.7 (5.6, 7.3) HR (95% CI) 0.40 (0.30, 0.53) p-value p<0.0001 Overall Response Rate (ORR)* ORR, % (95% CI) 67 (59, 75) 36 (29, 44) Complete response, % 4 1 Partial response, % 63 36 Duration of response (DOR)† Median (95% CI), months 10.1 (8.5, 13.9) 5.6 (4.4, 6.9) Figure 1: Kaplan-Meier Curve of PFS in Previously Untreated NSCLC Patients by BICR Assessment – Papillon Study
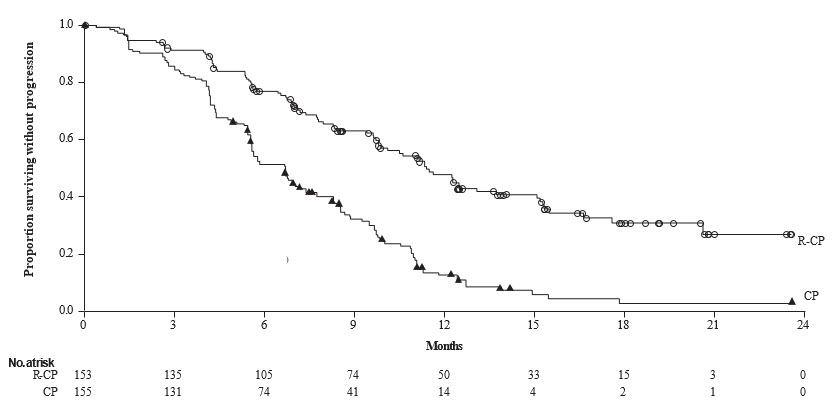
While OS results were immature at the current analysis, with 44% of pre-specified deaths for the final analysis reported, no trend towards a detriment was observed. Seventy-five (48%) of the treated patients crossed over from the carboplatin and pemetrexed arm after confirmation of disease progression to receive RYBREVANT as a single agent.
14.2 Previously Treated NSCLC with Exon 20 Insertion Mutations-CHRYSALIS
The efficacy of RYBREVANT was evaluated in patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations in a multicenter, open-label, multi-cohort clinical trial (CHRYSALIS, NCT02609776). The study included patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations whose disease had progressed on or after platinum-based chemotherapy. Patients with untreated brain metastases and patients with a history of ILD requiring treatment with prolonged steroids or other immunosuppressive agents within the last 2 years were not eligible for the study.
In the efficacy population, EGFR exon 20 insertion mutation status was determined by prospective local testing using tissue (94%) and/or plasma (6%) samples. Of the 81 patients with EGFR exon 20 insertion mutations identified by local testing, plasma samples from 78/81 (96%) patients were tested retrospectively using Guardant360 ®CDx, identifying 62/78 (79%) samples with an EGFR exon 20 insertion mutation; 16/78 (21%) samples did not have an EGFR exon 20 insertion mutation identified.
Patients received RYBREVANT at 1050 mg (for patient baseline body weight < 80 kg) or 1400 mg (for patient baseline body weight ≥80 kg) once weekly for 4 weeks, then every 2 weeks thereafter until disease progression or unacceptable toxicity. The major efficacy outcome measure was overall response rate (ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST v1.1) as evaluated by Blinded Independent Central Review (BICR). An additional efficacy outcome measure was duration of response (DOR) by BICR.
The efficacy population included 81 patients with NSCLC with EGFR exon 20 insertion mutation with measurable disease who were previously treated with platinum-based chemotherapy. The median age was 62 (range: 42 to 84) years, 59% were female; 49% were Asian, 37% were White, 2.5% were Black; 74% had baseline body weight <80 kg; 95% had adenocarcinoma; and 46% had received prior immunotherapy. The median number of prior therapies was 2 (range: 1 to 7). At baseline, 67% had Eastern Cooperative Oncology Group (ECOG) performance status of 1; 53% never smoked; all patients had metastatic disease; and 22% had previously treated brain metastases.
Efficacy results are summarized in Table 14.
Table 14: Efficacy Results for CHRYSALIS Prior Platinum-based Chemotherapy Treated
(N=81)Based on Kaplan-Meier estimates.
NE=Not Estimable, CI=confidence interval.Overall Response Rate(95% CI) 40% (29%, 51%) Complete response (CR) 3.7% Partial response (PR) 36% Duration of Response (DOR) Median, months (95% CI), months 11.1 (6.9, NE) Patients with DOR ≥6 months 63% - 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Infusion-Related Reactions
Advise patients that RYBREVANT can cause infusion-related reactions, the majority of which may occur with the first infusion. Advise patients to alert their healthcare provider immediately for any signs or symptoms of infusion-related reactions [see Warnings and Precautions (5.1)] .
Interstitial Lung Disease/Pneumonitis
Advise patients of the risks of interstitial lung disease (ILD)/pneumonitis. Advise patients to immediately contact their healthcare provider for new or worsening respiratory symptoms [see Warnings and Precautions (5.2)] .
Dermatologic Adverse Reactions
Advise patients of the risk of dermatologic adverse reactions. Advise patients to limit direct sun exposure, to use broad spectrum UVA/UVB sunscreen, and to wear protective clothing during treatment with RYBREVANT [see Warnings and Precautions (5.3)] . Advise patients to apply alcohol free emollient cream to dry skin.
Ocular Toxicity
Advise patients of the risk of ocular toxicity. Advise patients to contact their ophthalmologist if they develop eye symptoms and advise discontinuation of contact lenses until symptoms are evaluated [see Warnings and Precautions (5.4)] .
Paronychia/Nail Toxicity
Advise patients of the risk of paronychia. Advise patients to contact their healthcare provider for signs or symptoms of paronychia [see Adverse Reactions (6.1)].
Embryo-Fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus, to use effective contraception during treatment with RYBREVANT and for 3 months after the last dose, and to inform their healthcare provider of a known or suspected pregnancy. [see Warnings and Precautions (5.5), Use in Specific Populations (8.1, 8.3)].
Lactation
Advise women not to breastfeed during treatment with RYBREVANT and for 3 months after the last dose [see Use in Specific Populations (8.2)] .
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
RYBREVANT ®(RYE–breh–vant)
(amivantamab-vmjw)
injection, for intravenous useThis Patient Information has been approved by the U.S. Food and Drug Administration. Revised: March/2024 What is RYBREVANT?
RYBREVANT is a prescription medicine used to treat adults:- in combination with carboplatin and pemetrexed as a first-line treatment for non-small cell lung cancer (NSCLC) that:
- has spread to other parts of the body (metastatic) or cannot be removed by surgery, and
- has a certain abnormal epidermal growth factor receptor "EGFR" gene(s)
- alone for the treatment of non-small cell lung cancer (NSCLC) that:
- has spread to other parts of the body (metastatic) or cannot be removed by surgery, and
- has a certain abnormal EGFR gene(s), whose disease has worsened on or after platinum-based chemotherapy.
It is not known if RYBREVANT is safe and effective in children.Before you receive RYBREVANT, tell your healthcare provider about all of your medical conditions, including if you: - have a history of lung or breathing problems
- are pregnant or plan to become pregnant. RYBREVANT can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider should do a pregnancy test before you start treatment with RYBREVANT.
- You should use effective birth control (contraception) during treatment and for 3 months after your last dose of RYBREVANT.
- Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with RYBREVANT.
- are breastfeeding or plan to breastfeed. It is not known if RYBREVANT passes into your breast milk. Do not breastfeed during treatment and for 3 months after your last dose of RYBREVANT.
How will I receive RYBREVANT? - RYBREVANT will be given to you by your healthcare provider by intravenous infusion into your vein.
- Your healthcare provider will decide the time between doses as well as how many treatments you will receive.
- Your healthcare provider will give you medicines before each dose of RYBREVANT to help reduce the risk of infusion-related reactions.
- RYBREVANT may be given in combination with the medicines carboplatin and pemetrexed. If you have any questions about these medicines, ask your healthcare provider.
- If you miss any appointments, call your healthcare provider as soon as possible to reschedule your appointment.
RYBREVANT can cause skin reactions. You should limit your time in the sun during and for 2 months after your treatment with RYBREVANT. Wear protective clothing and use sunscreen during treatment with RYBREVANT. What are the possible side effects of RYBREVANT?
RYBREVANT may cause serious side effects, including:- infusion-related reactions.Infusion-related reactions are common with RYBREVANT and can be severe or serious. Tell your healthcare provider right away if you get any of the following symptoms during your infusion of RYBREVANT:
- shortness of breath
- fever
- chills
- nausea
- flushing
- chest discomfort
- lightheadedness
- vomiting
- lung problems.RYBREVANT may cause lung problems that may lead to death. Symptoms may be similar to those symptoms from lung cancer. Tell your healthcare provider right away if you get any new or worsening lung symptoms, including shortness of breath, cough, or fever.
- skin problems.RYBREVANT may cause rash, itching, and dry skin. You may use alcohol-free moisturizing cream for dry skin. Tell your healthcare provider right away if you get any skin reactions. Your healthcare provider may treat you with a medicine(s) or send you to see a skin specialist (dermatologist) if you get skin reactions during treatment with RYBREVANT. See " What should I avoid while receiving RYBREVANT?"
- eye problems.RYBREVANT may cause eye problems. Tell your healthcare provider right away if you get symptoms of eye problems which may include:
- eye pain
- dry eyes
- eye redness
- blurred vision
- changes in vision
- itchy eyes
- excessive tearing
- sensitivity to light
Your healthcare provider may send you to see an eye specialist (ophthalmologist) if you get eye problems during treatment with RYBREVANT. You should not use contact lenses until your eye symptoms are checked by a healthcare provider. The most common side effects of RYBREVANT in combination with carboplatin and pemetrexed include: - rash
- infected skin around the nail
- sores in the mouth
- infusion-related reactions
- feeling very tired
- swelling of hands, ankles, feet, face, or all of your body
- constipation
- decreased appetite
- nausea
- COVID-19
- diarrhea
- vomiting
- changes in certain blood tests
The most common side effects of RYBREVANT when given alone: - rash
- infusion-related reactions
- infected skin around the nail
- muscle and joint pain
- shortness of breath
- nausea
- feeling very tired
- swelling of hands, ankles, feet, face, or all of your body
- sores in the mouth
- cough
- constipation
- vomiting
- changes in certain blood tests
Your healthcare provider may temporarily stop, decrease your dose or completely stop your treatment with RYBREVANT if you have serious side effects.
These are not all of the possible side effects of RYBREVANT.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about safe and effective use of RYBREVANT
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your healthcare provider or pharmacist for information about RYBREVANT that is written for health professionals.What are the ingredients of RYBREVANT?
Active ingredient: amivantamab-vmjw
Inactive ingredients: EDTA disodium salt dihydrate, L-histidine, L-histidine hydrochloride monohydrate, L-methionine, polysorbate 80, sucrose, and water for injection.
Product of Ireland
Manufactured by: Janssen Biotech, Inc., Horsham, PA 19044, USA. U.S. License Number 1864
For patent information: www.janssenpatents.com
© 2022 Janssen Pharmaceutical Companies
For more information, call 1-800-526-7736 (1-800-JANSSEN) or go to www.RYBREVANT.com. - in combination with carboplatin and pemetrexed as a first-line treatment for non-small cell lung cancer (NSCLC) that:
- PRINCIPAL DISPLAY PANEL - 7 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
RYBREVANT
amivantamab injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:57894-501 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMIVANTAMAB (UNII: 0JSR7Z0NB6) (AMIVANTAMAB - UNII:0JSR7Z0NB6) AMIVANTAMAB 350 mg Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) HISTIDINE (UNII: 4QD397987E) HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) METHIONINE (UNII: AE28F7PNPL) POLYSORBATE 20 (UNII: 7T1F30V5YH) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) Product Characteristics Color yellow (Colorlous to Pale Yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57894-501-01 1 in 1 CARTON 05/21/2021 1 NDC:57894-501-00 1 in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761210 05/21/2021 Labeler - Janssen Biotech, Inc. (099091753) Establishment Name Address ID/FEI Business Operations Janssen Pharmaceutical Sciences Unlimited Company 985639841 api manufacture(57894-501) , analysis(57894-501) Establishment Name Address ID/FEI Business Operations Cilag AG 483237103 label(57894-501) , manufacture(57894-501) , analysis(57894-501)


