Label: WYNZORA- calcipotriene and betamethasone dipropionate cream
- NDC Code(s): 73499-001-01, 73499-001-03
- Packager: MC2 Therapeutics Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use WYNZORA Cream safely and effectively. See full prescribing information for WYNZORA Cream.
WYNZORA® (calcipotriene and betamethasone dipropionate) cream, for topical use
Initial U.S. Approval: 2020INDICATIONS AND USAGE
WYNZORA Cream is a combination of calcipotriene, a vitamin D analog, and betamethasone dipropionate, a corticosteroid, indicated for the topical treatment of plaque psoriasis in patients 18 years of age and older. (1)
DOSAGE AND ADMINISTRATION
- Apply WYNZORA Cream to affected areas once daily for up to 8 weeks. (2)
- Discontinue therapy when control is achieved. (2)
- Do not use more than 100 g per week. (2)
- Do not use with occlusive dressings unless directed by a physician. (2)
- Avoid use on the face, groin, or axillae, or if skin atrophy is present at the treatment site. (2)
- Not for oral, ophthalmic, or intravaginal use. (2)
DOSAGE FORMS AND STRENGTHS
Cream: 0.005%/0.064% (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Hypercalcemia and Hypercalciuria: Hypercalcemia and hypercalciuria have been observed with use of topical calcipotriene. If either occurs, discontinue until parameters of calcium metabolism normalize. (5.1)
- Effects on Endocrine System: Can cause reversible hypothalamicpituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency during and after withdrawal of treatment. Risk factors include the use of high-potency topical corticosteroid, use over a large surface area, or to areas under occlusion, prolonged use, altered skin barrier, liver failure, and young age. Modify use should HPA axis suppression develop. (5.2)
- Ophthalmic Adverse Reactions: May increase the risk of cataracts and glaucoma. If visual symptoms occur, consider referral to an ophthalmologist. (5.5)
ADVERSE REACTIONS
The most common adverse reactions reported by more than 1% of subjects treated with WYNZORA Cream were upper respiratory infection, headache, and application site irritation. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact MC2 Therapeutics Ltd at 1-800-644-8240 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypercalcemia and Hypercalciuria
5.2 Effects on Endocrine System
5.3 Allergic Contact Dermatitis with Topical Corticosteroids
5.4 Allergic Contact Dermatitis with Topical Calcipotriene
5.5 Ophthalmic Adverse Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Apply WYNZORA Cream to affected areas once daily for up to 8 weeks. Rub in gently to ensure that the plaques are saturated with the cream.
Do not use more than 100 g per week.
Discontinue therapy when control is achieved.
Do not use:
- with occlusive dressings unless directed by a healthcare provider
- on the face, groin, or axillae, or if skin atrophy is present at the treatment site
WYNZORA Cream is not for oral, ophthalmic, or intravaginal use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypercalcemia and Hypercalciuria
Hypercalcemia and hypercalciuria have been observed with use of topical calcipotriene. If hypercalcemia or hypercalciuria develop, discontinue treatment until parameters of calcium metabolism have normalized.
5.2 Effects on Endocrine System
Hypothalamic-Pituitary-Adrenal Axis SuppressionWYNZORA Cream can cause reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for clinical glucocorticosteroid insufficiency. This may occur during treatment or upon withdrawal of treatment. Factors that predispose a patient to HPA axis suppression include the use of high-potency steroids, large treatment surface areas, prolonged use, use of occlusive dressings, altered skin barrier, liver failure, and young age.
Evaluation for HPA axis suppression may be done by using the adrenocorticotropic hormone (ACTH) stimulation test. If HPA axis suppression is documented, gradually withdraw WYNZORA Cream, reduce the frequency of application, or substitute with a less potent corticosteroid.
The following trial evaluated the effects of WYNZORA Cream on HPA axis suppression:
HPA axis suppression was evaluated in adult subjects (N=27) with extensive psoriasis (including scalp). Adrenal suppression was seen in 6 out of 26 subjects (23%) after 4 weeks of treatment, and in 3 out of 25 subjects (12%) after 8 weeks of treatment [see Clinical Pharmacology (12.2)].
Cushing's Syndrome and HyperglycemiaCushing's syndrome and hyperglycemia may occur due to the systemic effects of the topical corticosteroid. These complications generally occur after prolonged exposure to excessively large doses, especially of high-potency topical corticosteroids.
Additional Considerations for Endocrine Adverse Reactions
Pediatric patients may be more susceptible to systemic toxicity due to their larger skin surface to body mass ratios [see Use in Specific Populations (8.4)].
Use of more than one corticosteroid-containing product at the same time may increase total systemic corticosteroid exposure.
5.3 Allergic Contact Dermatitis with Topical Corticosteroids
Allergic contact dermatitis to a topical corticosteroid is usually diagnosed by observing a failure to heal rather than a clinical exacerbation. Such an observation should be corroborated with appropriate diagnostic patch testing.
5.4 Allergic Contact Dermatitis with Topical Calcipotriene
Allergic contact dermatitis has been observed with use of topical calcipotriene. Such an observation should be corroborated with appropriate diagnostic patch testing.
5.5 Ophthalmic Adverse Reactions
Use of topical corticosteroids, including WYNZORA Cream, may increase the risks of glaucoma and posterior subcapsular cataract. Cataracts and glaucoma have been reported with the postmarketing use of topical corticosteroid products [see Adverse Reactions (6.2)].
Avoid contact of WYNZORA Cream with eyes. WYNZORA Cream may cause eye irritation. Advise patients to report any visual symptoms and consider referral to an ophthalmologist for evaluation.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The rates of adverse reactions given below were reported in a randomized, multicenter, prospective, vehicle and active controlled clinical trial in adult subjects with plaque psoriasis. Subjects applied WYNZORA Cream, calcipotriene/betamethasone dipropionate topical suspension, 0.005%/0.064% or vehicle once daily for 8 weeks. The mean weekly dose of WYNZORA Cream was 33.8 g. A total of 342 subjects were treated with WYNZORA Cream, 337 with calcipotriene/betamethasone dipropionate topical suspension, 0.005%/0.064% and 115 with vehicle. The majority of subjects were White (87%) and male (62%). Approximately 72% were non-Hispanic/Latino. The mean age was 52 years and ages ranged from 18 to 89 years.
The most common adverse reactions reported by ≥1% of subjects treated with WYNZORA Cream and more frequently than vehicle are presented in Table 1 below.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of topical corticosteroids. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Postmarketing reports for local adverse reactions to topical corticosteroids included: atrophy, striae, telangiectasias, itching, dryness, hypopigmentation, perioral dermatitis, secondary infection, and miliaria.
Ophthalmic adverse reactions of cataracts, glaucoma, and increased intraocular pressure, have been reported during use of topical corticosteroids, including topical betamethasone products.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data with WYNZORA Cream are not sufficient to evaluate a drug-associated risk for major birth defects, miscarriages, or adverse maternal or fetal outcomes. Although there are no available data on use of the calcipotriene component in pregnant women, systemic exposure to calcipotriene after topical administration of WYNZORA Cream is likely to be low [see Clinical Pharmacology (12.3)].
Observational studies suggest an increased risk of having low birthweight infants with the maternal use of potent or very potent topical corticosteroids (see Data). Advise pregnant women that WYNZORA Cream may increase the potential risk of having a low birth weight infant and to use WYNZORA Cream on the smallest area of skin and for the shortest duration possible.
In animal reproduction studies, oral administration of calcipotriene to pregnant rats during the period of organogenesis resulted in an increased incidence of minor skeletal abnormalities, including enlarged fontanelles and extra ribs (see Data). Oral administration of calcipotriene to pregnant rabbits during the period of organogenesis had no apparent effects on embryo-fetal development. Subcutaneous administration of betamethasone dipropionate to pregnant rats and rabbits during the period of organogenesis resulted in fetal toxicity, including fetal deaths, reduced fetal weight, and fetal malformations (cleft palate and crooked or short tail) (see Data). The available data do not allow the calculation of relevant comparisons between the systemic exposures of calcipotriene and betamethasone dipropionate observed in animal studies to the systemic exposures that would be expected in humans after topical use of WYNZORA Cream.
The background risk of major birth defects and miscarriage in the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
Available observational studies in pregnant women did not identify a drug-associated risk of major birth defects, preterm delivery, or fetal mortality with the use of topical corticosteroids of any potency. However, when the dispensed amount of potent or very potent topical corticosteroids exceeded 300 g during the entire pregnancy, maternal use was associated with an increased risk of low birth weight in infants.
Animal Data
Embryo-fetal development studies with calcipotriene were performed by the oral route in rats and rabbits. Pregnant rats received dosages of 0, 6, 18, or 54 mcg/kg/day (0, 36, 108, and 324 mcg/m2 /day, respectively) on days 6-15 of gestation (the period of organogenesis). There were no apparent effects on maternal survival, behavior, or body weight gain, no effects on litter parameters, and no effects on the incidence of major malformations in fetuses. Fetuses from dams dosed at 54 mcg/kg/day exhibited a significantly increased incidence of minor skeletal abnormalities, including enlarged fontanelles and extra ribs.
Pregnant rabbits were dosed daily with calcipotriene at exposures of 0, 4, 12, or 36 mcg/kg/day (0, 48, 144, and 432 mcg/m2 /day, respectively) on days 6-18 of gestation (the period of organogenesis). Mean maternal body weight gain was reduced in animals dosed at 12 or 36 mcg/kg/day. The incidence of fetal deaths was increased in the group dosed at 36 mcg/kg/day; reduced fetal weight was also observed in this group. The incidence of major malformations among fetuses was not affected. An increase in the incidence of minor skeletal abnormalities, including incomplete ossification of sternebrae, pubic bones, and forelimb phalanges, was observed in the group dosed at 36 mcg/kg/day.
Embryo-fetal development studies with betamethasone dipropionate were performed via subcutaneous injection in mice and rabbits. Pregnant mice were administered doses of 0, 156, 625, or 2500 mcg/kg/day (0, 468, 1875, and 7500 mcg/m2 /day, respectively) on days 7 through 13 of gestation (the period of organogenesis). Betamethasone dipropionate induced fetal toxicity, including fetal deaths, reduced fetal weight, malformations (increased incidence of the cleft palate and crooked or short tail), and minor skeletal abnormalities (delayed ossification of vertebra and sternebrae). Fetal toxicity was observed at the lowest exposure that was evaluated (156 mcg/kg/day).
Pregnant rabbits were injected subcutaneously at dosages of 0, 0.625, 2.5, and 10 mcg/kg/day (0, 7.5, 30, and 120 mcg/m2 /day, respectively) on days 6 through 18 of gestation (the period of organogenesis). Betamethasone dipropionate induced fetal toxicity, including fetal deaths, reduced fetal weight, external malformations (including malformed ears, cleft palate, umbilical hernia, kinked tail, club foot, and club hand), and skeletal malformations (including absence of phalanges of the first digit and cranial dysplasia) at dosages of 2.5 mcg/kg/day and above.
Calcipotriene was evaluated for effects on peri- and post-natal development when orally administered to pregnant rats at dosages of 0, 6, 18 or 54 mcg/kg/day (0, 36, 108, and 324 mcg/m2 /day, respectively) from gestation day 15 through day 20 postpartum. No remarkable effects were observed on any parameter, including survival, behavior, body weight, litter parameters, or the ability to nurse or rear pups.
Betamethasone dipropionate was evaluated for effects on peri- and post-natal development when orally administered to pregnant rats at dosages of 0, 100, 300, and 1000 mcg/kg/day (0, 600, 1800, and 6000 mcg/m2 /day, respectively) from gestation day 6 through day 20 postpartum. Mean maternal body weight was significantly reduced on gestation day 20 in animals dosed at 300 and 1000 mcg/kg/day. The mean duration of gestation was slightly, but statistically significantly, increased at 100, 300, and 1000 mcg/kg/day. The mean percentage of pups that survived to day 4 was reduced in relation to dosage. On lactation day 5, the percentage of pups with a reflex to right themselves when placed on their back was significantly reduced at 1000 mcg/ kg/day. No effects on the ability of pups to learn were observed, and the ability of the offspring of treated rats to reproduce was not affected.
8.2 Lactation
Risk Summary
There is no information regarding the presence of topically administered calcipotriene and betamethasone dipropionate in human milk, the effects on the breastfed infant, or the effects on milk production. Concentrations of calcipotriene in plasma are low after topical administration, and therefore, concentrations in human milk are likely to be low [see Clinical Pharmacology (12.3)].
It is not known whether topically administered calcipotriene or corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for WYNZORA Cream and any potential adverse effects on the breastfed child from WYNZORA Cream or from the underlying maternal condition.
Clinical Considerations
To minimize potential exposure to the breastfed infant via breast milk, use WYNZORA Cream on the smallest area of skin and for the shortest duration possible while breastfeeding. Advise breastfeeding women not to apply WYNZORA Cream directly to the nipple and areola to avoid direct infant exposure.
8.4 Pediatric Use
Safety and effectiveness of the use of WYNZORA Cream in adolescents and pediatric patients under the age of 18 years have not been established.
Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of systemic toxicity when treated with topical corticosteroids. Pediatric patients are, therefore, also at greater risk of HPA axis suppression and adrenal insufficiency with the use of topical corticosteroids including WYNZORA Cream [see Clinical Pharmacology (12.2)].
Systemic toxicities such as Cushing's syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in pediatric patients, especially those with prolonged exposure to large doses of high potency topical corticosteroids. Local adverse reactions including striae have also been reported with use of topical corticosteroids in pediatric patients.
8.5 Geriatric Use
The trial included 66 subjects ≥ 65 years of age treated with WYNZORA Cream.
No overall differences in safety or effectiveness of WYNZORA Cream were observed between these subjects and younger subjects. All other reported clinical experience has not identified any differences in response between elderly and younger patients. However, greater sensitivity of some older individuals cannot be ruled out.
-
11 DESCRIPTION
WYNZORA (calcipotriene and betamethasone dipropionate) Cream contains anhydrous calcipotriene and betamethasone dipropionate intended for topical use.
Calcipotriene is a synthetic vitamin D3 analog.
Chemically, calcipotriene is (5Z,7E,22E,24S)-24-Cyclopropyl-9,10-secochola-5,7,10(19),22 tetraene-1α,3β,24-triol, with the empirical formula C27H40O3, a molecular weight of 412.6, and the following structural formula:
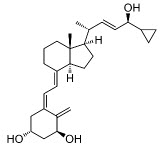
Calcipotriene is a white or almost white powder. It is insoluble in water, freely soluble in ethanol and slightly soluble in methylene chloride.
Betamethasone dipropionate is a synthetic corticosteroid.
Betamethasone dipropionate has the chemical name Pregna-1,4-diene-3,20-dione,9-fluoro-11-hydroxy-16-methyl-17,21-bis(1-oxypropoxy)-,(11β,16β), with the empirical formula C28H37FO7, a molecular weight of 504.6, and the following structural formula:
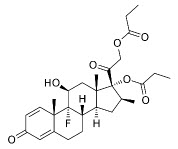
Betamethasone dipropionate is a white to almost white crystalline powder. It is practically insoluble in water, freely soluble in acetone and in methylene chloride, sparingly soluble in alcohol.
Each gram of WYNZORA Cream contains 50 mcg of calcipotriene and 0.644 mg of betamethasone dipropionate (equivalent to 0.5 mg of betamethasone). WYNZORA Cream also contains the following inactive ingredients: isopropyl myristate, mineral oil, medium-chain triglycerides, isopropyl alcohol, polyoxyl lauryl ether, poloxamer (407), polyoxyl 40 hydrogenated castor oil, carbomer interpolymer (type A), butylated hydroxyanisole, trolamine, dibasic sodium phosphate, heptahydrate, monobasic sodium phosphate, monohydrate, alpha-tocopherol and purified water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
WYNZORA Cream combines the pharmacological effects of calcipotriene as a synthetic vitamin D3 analog and betamethasone dipropionate as a synthetic corticosteroid. However, while their pharmacologic and clinical effects are known, the exact mechanisms of their actions in plaque psoriasis are unknown.
12.2 Pharmacodynamics
Vasoconstriction:
In a vasoconstrictor trial in healthy subjects, the skin blanching response of WYNZORA Cream was consistent with a mid-strength corticosteroid when compared with other topical corticosteroids. However, similar blanching scores do not necessarily imply therapeutic equivalence.
Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression:
HPA axis suppression was evaluated in adult subjects with extensive psoriasis involving 20-30% of the body surface area (including scalp). Treatment consisted of once daily application of WYNZORA Cream to the body (including scalp involvement in 75% of the subjects) for up to 8 weeks. Adrenal suppression indicated by 30-minute post-stimulation serum cortisol level of ≤18 mcg/dL was seen in 6 out of 26 subjects (23%) after 4 weeks of treatment, and in 3 out of 25 subjects (12%; one subject with continued suppression from Week 4 and two additional subjects) after 8 weeks of treatment.
There was no trend towards decreasing cortisol levels post-ACTH stimulation with increasing systemic concentration of betamethasone 17-propionate (B17P) measured as AUC0-7 or Cmax or increasing average weekly amount of WYNZORA Cream used.
Effects on Calcium Metabolism:
The effects on calcium metabolism of once daily application of WYNZORA Cream to the body (including scalp involvement in 75% of subjects) for up to 8 weeks were also examined and no cases of hypercalcemia and no clinically relevant changes in urine calcium were reported.
12.3 Pharmacokinetics
Absorption
The pharmacokinetics of WYNZORA Cream was investigated in adult subjects in the same study as described above [see Clinical Pharmacology (12.2)]. Their mean ± SD total body surface area involvement was 25 ± 5 % and 74% of the subjects presented with scalp involvement and with a mean ± SD scalp involvement of 52 ± 40 %. The mean ± SD weekly dose during the 8 weeks of treatment was 79 ± 30 g.
Plasma concentrations of calcipotriene and betamethasone dipropionate and their major metabolites were measured after 4 weeks and 8 weeks of once daily application of WYNZORA Cream.
In most samples, concentrations of the four analytes were below or close to lower limit of quantification (LOQ). One of 27 (4%) subjects had quantifiable levels of calcipotriene at Week 4 and the Cmax and AUC0-7 were 30 pg/mL and 229 pg*h/mL, respectively. For the major metabolite of calcipotriene, MC1080, 3 of 27 (11%) subjects had quantifiable levels at Week 4. The mean ± SD Cmax and AUC0-7 were 30 ± 4 pg/mL and 224 ± 16 pg*h/mL, respectively. No subjects had quantifiable levels of calcipotriene or MC1080 at Week 8.
There were 3 of 27 subjects (11%) with quantifiable levels of betamethasone dipropionate at Week 4. The mean ± SD of Cmax and AUC0-7 were 22 ± 9 pg/mL and 160 ± 36 pg*h/mL, respectively. The major metabolite of betamethasone dipropionate, B17P, was quantifiable in 13 of 27 subjects (48%) at Week 4. The mean ± SD Cmax and AUC0-7 were 96 ± 234 pg/mL and 419 ± 646 pg*h/mL, respectively. No subjects had quantifiable levels of betamethasone dipropionate at Week 8, whereas 7 of 19 (37%) subjects had quantifiable levels of B17P. The mean ± SD Cmax and AUC0-7 were 31 ± 29 pg/mL and 205 ± 142 pg*h/mL, respectively.
Metabolism
Calcipotriene:
Calcipotriene metabolism following systemic uptake is rapid and occurs in the liver. The primary metabolites of calcipotriene are less potent than the parent compound.
Calcipotriene is metabolized to MC1046 (the α,ß-unsaturated ketone analog of calcipotriene), which is metabolized further to MC1080 (a saturated ketone analog). MC1080 is the major metabolite in plasma. MC1080 is slowly metabolized to calcitroic acid.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
When calcipotriene was applied topically to mice for up to 24 months at dosages of 3, 10, and 30 mcg/kg/day (corresponding to 9, 30, and 90 mcg/m2/day), no significant changes in tumor incidence were observed when compared to control.
A 104-week oral carcinogenicity study was conducted with calcipotriene in male and female rats at doses of 1, 5 and 15 mcg/kg/day (corresponding to dosages of approximately 6, 30, and 90 mcg/m2/day). Beginning week 71, the dosage for high-dose animals of both genders was reduced to 10 mcg/kg/day (corresponding to a dosage of approximately 60 mcg/m2/day). A treatment-related increase in benign C-cell adenomas was observed in the thyroid of females that received 15 mcg/kg/day. A treatment-related increase in benign pheochromocytomas was observed in the adrenal glands of males that received 15 mcg/kg/day. No other statistically significant differences in tumor incidence were observed when compared to control. The relevance of these findings to patients is unknown.
When betamethasone dipropionate was applied topically to CD-1 mice for up to 24 months at dosages approximating 1.3, 4.2, and 8.5 mcg/kg/day in females, and 1.3, 4.2, and 12.9 mcg/kg/day in males (corresponding to dosages of up to approximately 26 mcg/m2/day and 39 mcg/m2/day, in females and males, respectively), no significant changes in tumor incidence were observed when compared to control.
When betamethasone dipropionate was administered via oral gavage to male and female Sprague Dawley rats for up to 24 months at dosages of 20, 60, and 200 mcg/kg/day (corresponding to dosages of approximately 120, 360, and 1200 mcg/m2/day), no significant changes in tumor incidence were observed when compared to control.
Calcipotriene did not elicit any genotoxic effects in the Ames mutagenicity assay, the mouse lymphoma TK locus assay, the human lymphocyte chromosome aberration test, or the mouse micronucleus test. Betamethasone dipropionate did not elicit any genotoxic effects in the Ames mutagenicity assay, the mouse lymphoma TK locus assay, or in the rat micronucleus test.
Studies in rats with oral doses of up to 54 mcg/kg/day (324 mcg/m2/day) of calcipotriene indicated no impairment of fertility or general reproductive performance. Studies in male rats at oral doses of up to 200 mcg/kg/day (1200 mcg/m2/day), and in female rats at oral doses of up to 1000 mcg/kg/day (6000 mcg/m2/day), of betamethasone dipropionate indicated no impairment of fertility.
-
14 CLINICAL STUDIES
The safety and efficacy of WYNZORA Cream were evaluated in a randomized, multicenter, vehicle and active-comparator controlled, clinical trial (NCT03308799) in adult subjects with mild to moderate plaque psoriasis. In the trial, 794 subjects were randomized to 1 of 3 treatment groups: WYNZORA Cream, vehicle cream, or calcipotriene/betamethasone dipropionate topical suspension, 0.005%/0.064%. The majority of subjects in the trial (81.7%) had disease of moderate severity at baseline, while 18.3% of subjects had disease of mild severity. Disease severity was determined by a 5-grade Physician's Global Assessment (PGA) scale.
The primary efficacy endpoint was the proportion of subjects with treatment success at Week 8. Treatment success was defined as at least a 2-grade improvement from baseline in the PGA score and an PGA score equating to "clear" or "almost clear". Other evaluated outcomes included reduction in itch as defined by at least a 4-point improvement in the 11-point peak pruritus numeric rating scale (NRS) from baseline to Week 4. Table 2 presents the primary efficacy results.
Table 2: Primary Efficacy Outcome at Week 8 WYNZORA Cream
(N=342)Vehicle Cream
(N=115)PGA of Clear or Almost Clear And ≥2-grade Improvement 37.4% 3.7% Difference from Vehicle
(95% CI)33.7%
(27.4%, 40.0%)WYNZORA Cream was non-inferior to calcipotriene/betamethasone dipropionate topical suspension, 0.005%/0.064% for the primary endpoint of treatment success at Week 8 [Difference (95% CI): 14.6% (7.6%, 21.6%)].
Among subjects who had at least a peak pruritus NRS score of 4 at baseline, there was a higher percentage of subjects that achieved at least a 4-point improvement from baseline on the peak pruritus NRS score at Week 4 in the WYNZORA Cream group compared to the vehicle cream group (60.3% vs. 21.4%).
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Administration Instructions
- Instruct patients not to use more than 100 grams per week.
- Instruct patients to discontinue therapy when control is achieved unless directed otherwise by the healthcare provider.
- Advise patients to avoid use of WYNZORA Cream on the face, underarms, groin or eyes.
- Advise patients not to occlude the treatment area with a bandage or other covering unless directed by the healthcare provider.
- Instruct patients to wash hands after application.
Local Reactions and Skin Atrophy
Advise patients that local reactions and skin atrophy are more likely to occur with occlusive use, prolonged use or use of higher potency corticosteroids.
Hypercalcemia and Hypercalciuria
Advise patients that hypercalcemia and hypercalciuria may occur with the use of WYNZORA Cream [see Warnings and Precautions (5.1)].
HPA Axis Suppression, Cushing's Syndrome, and Hyperglycemia
Advise patients that WYNZORA Cream can cause HPA access suppression, Cushing's syndrome, and/or hyperglycemia [see Warnings and Precautions (5.2)].
Ophthalmic Adverse Reactions
Advise patients to avoid contact of WYNZORA Cream with eyes and to report any visual symptoms [see Warnings and Precautions (5.5)].
Possible Avoidance of Other Products Containing Calcipotriene or a Corticosteroid
Instruct patients not to use other products containing calcipotriene or a corticosteroid with WYNZORA Cream without first talking to the healthcare provider.
Pregnancy and Lactation
- Advise pregnant women that WYNZORA Cream may increase the potential risk of having a low birth weight infant and to use WYNZORA Cream on the smallest area of skin and for the shortest duration possible [see Use in Specific Populations (8.1)].
- Advise breastfeeding women not to apply WYNZORA Cream directly to the nipple and areola to avoid direct infant exposure [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration Issued: 11/2023 PATIENT INFORMATION
WYNZORA® (win-ZOR-uh)
(calcipotriene and betamethasone dipropionate)
CreamImportant information: WYNZORA® Cream is for use on the skin only (topical use only). Do not get WYNZORA® Cream near or in your mouth, eyes, or vagina. There are other medicines that contain the same medicine that is in WYNZORA® Cream and are used to treat plaque psoriasis. Do not use other products containing calcipotriene or a corticosteroid medicine with WYNZORA® Cream without talking to your healthcare provider first. What is WYNZORA® Cream?
WYNZORA® Cream is a prescription medicine used on the skin (topical) to treat plaque psoriasis in people 18 years of age and older.
It is not known if WYNZORA® Cream is safe and effective in children under 18 years of age.Before using WYNZORA® Cream, tell your healthcare provider about all of your medical conditions, including if you: - have a calcium metabolism disorder.
- have thinning skin (atrophy) at the treatment site.
- are pregnant or plan to become pregnant. It is not known if WYNZORA® Cream will harm your unborn baby. WYNZORA® Cream may increase your chance of having a low birth weight baby. If you use WYNZORA® Cream during pregnancy, use WYNZORA® Cream on the smallest area of the skin and for the shortest time needed.
- are breastfeeding or plan to breastfeed. It is not known if WYNZORA® Cream passes into your breast milk. Breastfeeding women should use WYNZORA® Cream on the smallest area of the skin and for the shortest time needed. Do not apply WYNZORA® Cream directly to the nipple and areola to avoid contact with your baby.
How should I use WYNZORA® Cream? - Use WYNZORA® Cream exactly as prescribed by your healthcare provider.
- Your healthcare provider should tell you how much WYNZORA® Cream to use and where to use it.
- Apply WYNZORA® Cream to affected areas 1 time a day for up to 8 weeks. You should stop treatment when your plaque psoriasis is under control unless your healthcare provider gives you other instructions.
- You should not use more than 100 grams of WYNZORA® Cream in 1 week.
- Do not use WYNZORA® Cream longer than prescribed. Using too much WYNZORA® Cream, or using it too often or for too long, can increase your risk for having serious side effects.
- Do not use WYNZORA® Cream in the mouth, eyes, or vagina.
- Do not use WYNZORA® Cream on your face, groin, or armpits, or if you have thinning of your skin (atrophy) at the treatment site.
- If you accidentally get WYNZORA® Cream on your face or in your eyes wash the area with water right away.
- Wash your hands well after applying WYNZORA® Cream.
- Do not bandage or cover the treated skin area, unless instructed by your healthcare provider.
- Remove the cap and check that the aluminum seal covers the opening on the top of the tube before the first use. To break the seal, turn the cap over and poke a hole through the seal.
- Gently rub WYNZORA® Cream all the way in to make sure that the plaques are well covered with the cream.
What are the possible side effects of WYNZORA® Cream?
WYNZORA® Cream may cause serious side effects, including:- Too much calcium in your blood or urine. Your healthcare provider may tell you to stop or temporarily stop treatment with WYNZORA® Cream if you have too much calcium in your blood or urine.
- WYNZORA® Cream can pass through your skin. Too much WYNZORA® Cream passing through your skin can cause your adrenal glands to stop working properly. Your healthcare provider may do blood tests to check for adrenal gland problems. Your healthcare provider may tell you to stop or temporarily stop treatment with WYNZORA® Cream.
- Cushing's syndrome, a condition that happens when your body is exposed to large amounts of the hormone cortisol.
- High blood sugar (hyperglycemia).
- Skin problems. Tell your healthcare provider if you have any skin problems, including:
- thinning of your skin
- burning
- inflammation
- itching
- irritation
- dryness
- changes in skin color
- redness
- infection
- raised bumps on your skin
- Eye problems. Using WYNZORA® Cream may increase your chance of getting cataracts and glaucoma. Do not get WYNZORA® Cream in your eyes because it may cause eye irritation. Tell your healthcare provider if you have blurred vision or other vision problems during treatment with WYNZORA® Cream.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1- 800-FDA-1088.How should I store WYNZORA® Cream? - Store WYNZORA® Cream at room temperature between 68°F to 77°F (20°C to 25°C) with the cap on the tube tightly closed.
- Do not freeze and protect WYNZORA® Cream from light and excessive heat.
- Keep WYNZORA® Cream out of the light.
- Throw away (discard) unused WYNZORA® Cream 6 months after it has been opened.
General information about WYNZORA® Cream
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use WYNZORA® Cream for a condition for which it was not prescribed. Do not give WYNZORA® Cream to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about WYNZORA® Cream that is written for health professionals.What are the ingredients in WYNZORA® Cream?
Active ingredients: calcipotriene and betamethasone dipropionate
Inactive ingredients: isopropyl myristate, mineral oil, medium-chain triglycerides, isopropyl alcohol, polyoxyl lauryl ether, poloxamer (407), polyoxyl 40 hydrogenated castor oil, carbomer interpolymer (type A), butylated hydroxyanisole, trolamine, dibasic sodium phosphate, heptahydrate, monobasic sodium phosphate, monohydrate, alpha-tocopherol and purified water.
Manufactured for: MC2 Therapeutics, Ltd. 1A Guildford Business Park, Guildford, GU2 8XG, United Kingdom.
For more information, go to www.wynzora.com or call 1-800-644-8240 - PRINCIPAL DISPLAY PANEL - 60 g Tube Carton
- PRINCIPAL DISPLAY PANEL - 5 g Tube Carton
-
INGREDIENTS AND APPEARANCE
WYNZORA
calcipotriene and betamethasone dipropionate creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73499-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Calcipotriene (UNII: 143NQ3779B) (Calcipotriene - UNII:143NQ3779B) Calcipotriene 50 ug in 1 g Betamethasone dipropionate (UNII: 826Y60901U) (Betamethasone - UNII:9842X06Q6M) Betamethasone 64 mg in 1 g Inactive Ingredients Ingredient Name Strength Isopropyl myristate (UNII: 0RE8K4LNJS) Mineral oil (UNII: T5L8T28FGP) Medium-chain triglycerides (UNII: C9H2L21V7U) Isopropyl alcohol (UNII: ND2M416302) Laureth-4 (UNII: 6HQ855798J) Poloxamer 407 (UNII: TUF2IVW3M2) Polyoxyl 40 hydrogenated castor oil (UNII: 7YC686GQ8F) Carbomer interpolymer type A (Allyl Sucrose Crosslinked) (UNII: 59TL3WG5CO) Butylated hydroxyanisole (UNII: REK4960K2U) Trolamine (UNII: 9O3K93S3TK) Sodium phosphate, dibasic, heptahydrate (UNII: 70WT22SF4B) Sodium phosphate, monobasic, monohydrate (UNII: 593YOG76RN) .Alpha.-Tocopherol, Dl- (UNII: 7QWA1RIO01) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73499-001-01 1 in 1 CARTON 06/30/2021 1 60 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:73499-001-03 10 in 1 CARTON 06/30/2021 2 5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213422 06/30/2021 Labeler - MC2 Therapeutics Ltd (345388560)




