Label: VISTASEAL- human fibrinogen, human thrombin kit
- NDC Code(s): 61953-0011-1, 61953-0012-1, 61953-0013-1, 61953-0014-1
- Packager: GRIFOLS USA, LLC
- Category: PLASMA DERIVATIVE
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VISTASEAL™ safely and effectively. See full prescribing information for VISTASEAL.
VISTASEAL™ [Fibrin Sealant (Human)]
Frozen solutions of fibrinogen and thrombin
For Topical Use Only
Initial U.S. Approval: 2017
INDICATIONS AND USAGE
VISTASEAL™ is indicated as an adjunct to hemostasis for mild to moderate bleeding in adults undergoing surgery when control of bleeding by standard surgical techniques (such as suture, ligature, and cautery) is ineffective or impractical. VISTASEAL is effective in heparinized patients. (1)
DOSAGE AND ADMINISTRATION
For Topical Use Only.
- After thawing, use VISTASEAL within 48 hours if stored at 2 ºC - 8 ºC [36 ºF - 46 ºF], or within 24 hours if stored at room temperature (20 °C ‑ 25 °C [68 ºF - 77 ºF]), if it remains sealed in the original packaging. (2.2,16)
- Prior to applying VISTASEAL, use standard techniques (e.g., intermittent application of compresses, swabs, use of suction devices) to dry the surface area of the target bleeding site. (2.3)
- Apply VISTASEAL by dripping or spraying. When applying VISTASEAL using a spray device, ensure that the distance from the tissue is within the recommended ranges for the applicator tip used. (2.3, 2.4)
- Apply a sufficient volume of VISTASEAL to entirely cover the intended application area with a thin layer. (2.1, 2.4)
DOSAGE FORMS AND STRENGTHS
VISTASEAL is supplied as a kit consisting of two separate packages: (3)
- A package containing one syringe each of human fibrinogen 80 mg/mL (component 1) and human thrombin 500 IU/mL (component 2) sterile frozen solutions which are assembled in a syringe holder.
- A package containing a VISTASEAL Dual Applicator with two additional Airless Spray Tips.
VISTASEAL is available in the following package sizes: (3)
Package size
(Total volume)Human
fibrinogenHuman
thrombin2 mL 1 mL 1 mL 4 mL 2 mL 2 mL 6 mL 3 mL 3 mL 10 mL 5 mL 5 mL CONTRAINDICATIONS
- Do not inject directly into the circulatory system. (4)
- Do not use for the treatment of severe or brisk arterial bleeding.
- Do not use in patients with history of anaphylaxis or severe systemic reactions to human blood products. (4)
- Do not use VISTASEAL for spraying unless the minimum recommended distance from the applicator tip to the bleeding site can be achieved. (2.3,4)
WARNINGS AND PRECAUTIONS
- Thromboembolic events may occur if VISTASEAL is administered intravascularly. (5.1)
- Hypersensitivity reactions can occur. (5.2)
- May carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. (5.3)
ADVERSE REACTIONS
The most common adverse reactions (reported in >1% of clinical trial subjects) were nausea and procedural pain. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Grifols Therapeutics LLC at 1-800-520-2807 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Preparation and Handling
2.3 Administration
2.4 Application precautions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Thrombosis
5.2 Hypersensitivity
5.3 Transmissible Infectious Agents
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenicity, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For topical use only.
2.1 Dosage
Individualize application of the fibrin sealant. Individual doses typically ranged from 0.3 to 18.0 mL in the clinical studies. Larger volumes may be required for surgical procedures other than those included in the clinical studies.
The approximate surface area coverage for each VISTASEAL package size is provided in Table 1.
Table 1. Surface Area Coverage VISTASEAL package size Surface area coverage (cm2)
Application by dripping or spray
(1 mm thick layer)2 mL 16 - 22 4 mL 32 - 44 6 mL 48 - 66 10 mL 80 - 110 Dose depends on variables including, but not limited to, the type of surgical intervention, the size of the area, the intended application method, and the number of applications.
Apply a sufficient volume of VISTASEAL to entirely cover the intended application area with a thin layer. Repeat the application if necessary.
2.2 Preparation and Handling
Prepare and administer the product only according to the instructions and with the recommended devices.
Remove carton from freezer, open it and take out the two blisters.
Place the blister containing the VISTASEAL Dual Applicator at room temperature until the VISTASEAL Fibrin Sealant (Human) is ready to use.Room Temperature Thawing (preferred method)
Thaw blister with VISTASEAL pre-filled syringes at room temperature using the following steps:
- Place the blister containing the syringe holder with pre-filled syringes on a surface at room temperature (20 ºC - 25 ºC, [68 - 77 ºF])
for approximately 70 minutes for the 2 mL and the 4 mL package sizes
for approximately 90 minutes for the 6 mL and the 10 mL package sizes
After thawing, it is not necessary to warm the product for its use.
After thawing, the solutions must be clear to slightly opalescent and colorless to pale yellow.
Do not use solutions that are cloudy or have deposits.
Post-Thawing Storage:
After thawing, the kit containing the VISTASEAL syringe holder with pre-filled syringes and Dual Applicator can be stored before use for not more than 48 hours in the refrigerator at 2 ‑ 8 ºC [36 - 46 ºF] or 24 hours at room temperature (20 - 25 °C [68 - 77 ºF]) if it remains sealed in the original packaging. Once the blisters are opened, use VISTASEAL immediately during the surgery and discard any unused contents.
Once thawed, do not refreeze.
Transferring instructions:
- After thawing, remove the blister from the surface at room temperature or from the refrigerator at 2 - 8 °C [36 - 46 ºF].
- Open the blister and make the VISTASEAL syringe holder with pre-filled syringes available to a second person for transfer to the sterile field. The outside of the blister should not come in contact with the sterile field. See Figure 1.
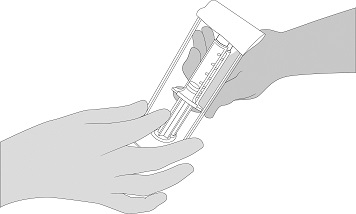
Figure 1
Sterile Water Bath (Quick Thawing)
Thaw VISTASEAL pre-filled syringes inside the sterile field in a sterile thermostatic water bath at a temperature not higher than 37 ºC [99 ºF] using the following steps:
NOTE: Once the VISTASEAL blisters are opened, use the product immediately during surgery. Use sterile technique to avoid the possibility of contamination due to improper handling, and follow the steps below accurately. Do not remove the syringe luer cap until thawing is complete and the Dual Applicator is ready to be attached.- Open the blister and make the VISTASEAL syringe holder with pre-filled syringes available to a second person for transfer to the sterile field. The outside of the blister should not come in contact with the sterile field. See Figure 1.
- Place the syringe holder with pre-filled syringes directly into the sterile water bath ensuring that it is completely immersed in the water. See Figure 2.
- At 37 ºC, the time needed is approximately 5 minutes for the 2 mL, 4 mL, 6 mL, and 10 mL package sizes, but must not be left at this temperature for longer than 10 minutes.
The temperature of the water bath must not exceed 37 ºC. - Dry the syringe holder with pre-filled syringes after thawing, using a sterile surgical gauze.
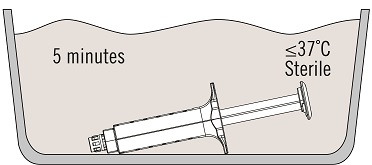
Figure 2
After thawing, the solutions must be clear to slightly opalescent and colorless to pale yellow.
Do not use solutions that are cloudy or have deposits.
Use VISTASEAL immediately during the surgery and discard any unused contents.
Connection instructions
- Open the blister and make the VISTASEAL Dual Applicator and two additional Airless Spray Tips available to a second person for transfer to the sterile field. The outside of the blister should not come in contact with the sterile field.
- Hold the VISTASEAL Fibrin Sealant (Human) syringe holder with syringe luer caps pointed upward. See Figure 3.
- Unscrew and discard the syringe luer cap of both fibrinogen and thrombin syringes. See Figure 3.
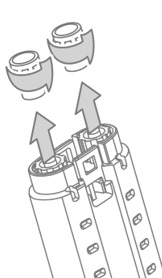
Figure 3
- Hold the syringe holder with the luers pointed upward. To remove air bubbles from syringes, strike gently the side of the syringe holder one or two times while keeping the syringe holder in an upright position and lightly depress the plunger to eject air. See Figure 4.
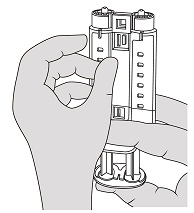
Figure 4
- Attach the Dual Applicator. See Figure 5.
NOTE: Do not depress plunger during attachment or prior to intended use because the two biologic components will pre-mix in the Airless Spray Tip, forming a fibrin clot that prevents dispensing. See Figure 6.
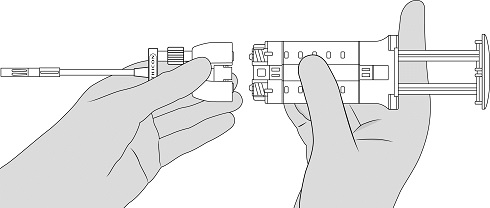
Figure 5
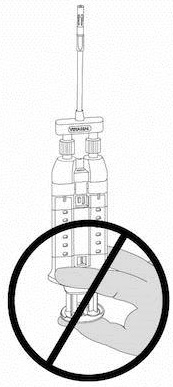
Figure 6
- Tighten luer locks and ensure the Dual Applicator is firmly attached. The device is now ready to use.
2.3 Administration
Apply VISTASEAL Fibrin Sealant (Human) using the syringe holder and plunger supplied.
Apply VISTASEAL Fibrin Sealant (Human) using the Dual Applicator provided with the product. Applicator tips cleared by the FDA for specific use with the VISTASEAL Fibrin Sealant (Human) may also be used. When using the provided Dual Applicator, follow the connection instructions in the above section for Preparation. When using other applicator tips, follow the instructions for use that are provided with the applicator tips.
Before administration of VISTASEAL Fibrin Sealant (Human):
- To prevent tissue adhesion at undesired sites, protect (cover) parts of the body outside the intended application area. [see Dosage and Administration (2.4) ]
- Use standard techniques (e.g., intermittent application of compresses, swabs, use of suction devices) to dry the surface area of the target bleeding site.
Application by spraying
- Grasp and bend the Dual Applicator to the desired position. Tip will retain its shape.
- Position the Airless Spray Tip at least 2 cm away from the target tissue. Apply firm even pressure to the plunger to spray the fibrin sealant. Increase distance accordingly to achieve desired coverage of the target area.
- If expression is stopped for any reason, change the Airless Spray Tip. To change the Airless Spray Tip, remove the device from the patient and unscrew the used Airless Spray Tip. See Figure 7. Place the used Airless Spray Tip away from the spare Airless Spray Tips. Wipe the end of the applicator using dry or moist sterile surgical gauze. Then, connect a new Airless Spray Tip provided in the package and ensure it is firmly connected before use.
NOTE: Red indicator will not be visible if Airless Spray Tip is properly connected. See Figure 8.
NOTE: Do not continue pushing the plunger in an attempt to clear the fibrin clot within the Airless Spray Tip; otherwise the applicator may become unusable.
NOTE: Do not trim the Dual Applicator to avoid exposing internal wire.
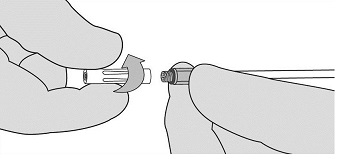
Figure 7
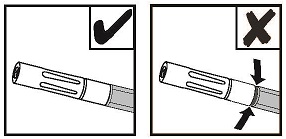
Figure 8
Application by dripping
- Remove the Airless Spray Tip portion of the spray and drip tip by unscrewing the Airless Spray Tip.
See Figure 7. - Grasp and bend the drip tip to the desired position. Tip will retain its shape.
- During dripping, keep the end of the drip tip as close to the tissue surface as possible without touching the tissue during application.
- Apply individual drops to the surface area to be treated. To prevent uncontrolled clotting, allow the drops to separate from each other and from the end of the drip tip.
NOTE: Do not reconnect a used drip tip after it has been removed from the adapter; otherwise a clot may form inside the drip tip and the applicator may become unusable.
2.4 Application precautions
- Before administration of VISTASEAL, protect (cover) parts of the body outside the desired application area to prevent tissue adhesion at undesired sites. [see Dosage and Administration (2.3)
- Apply VISTASEAL as a thin layer. Excessive clot thickness may negatively interfere with the product’s efficacy and the wound healing process. [see Dosage and Administration (2.1)]
- Only spray VISTASEAL if it is possible to accurately judge the distance from the spray tip to the tissue surface. [see Dosage and Administration (2.3)]
- No clinical data are available to support the use of this product in neurosurgery or application through a flexible endoscope for treatment of bleeding.
- Place the blister containing the syringe holder with pre-filled syringes on a surface at room temperature (20 ºC - 25 ºC, [68 - 77 ºF])
-
3 DOSAGE FORMS AND STRENGTHS
VISTASEAL is supplied as a kit consisting of two separate packages:
- A package containing one syringe each of human fibrinogen 80 mg/mL (component 1) and human thrombin 500 IU/mL (component 2) sterile frozen solutions which are assembled on a syringe holder.
- A package containing a Dual Applicator with two additional Airless Spray Tips.
The available package sizes of VISTASEAL are shown in Table 2.
Table 2. VISTASEAL Package Sizes Package size (Total volume) Human fibrinogen Human thrombin 2 mL 1 mL 1 mL 4 mL 2 mL 2 mL 6 mL 3 mL 3 mL 10 mL 5 mL 5 mL -
4 CONTRAINDICATIONS
- Do not inject directly into the circulatory system. [see Warnings and Precautions (5.1)]
- Do not use for the treatment of severe or brisk arterial bleeding. In these situations, blood flow will wash away VISTASEAL and prevent hemostasis.
- Do not use VISTASEAL in patients known to have anaphylactic or severe systemic hypersensitivity reactions to the administration of human blood products. [see Warnings and Precautions (5.2)]
- Do not use VISTASEAL for spraying unless the minimum recommended distance from the applicator tip to the bleeding site can be achieved. [see Dosage and Administration (2.3)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Thrombosis
Life-threatening thromboembolic complications may occur if VISTASEAL is administered intravascularly.
5.2 Hypersensitivity
Allergic-type hypersensitivity reactions are possible. Signs of hypersensitivity reactions include hives, generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis. If these symptoms occur, discontinue the administration of VISTASEAL immediately. Treat the reaction accordingly.
5.3 Transmissible Infectious Agents
Because VISTASEAL is made from human plasma, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and theoretically, the Creutzfeldt-Jakob (CJD) agent. This also applies to unknown or emerging viruses and other pathogens. All suspected infections related to this product should be reported by the physician or other healthcare provider to Grifols Therapeutics LLC at 1-800-520-2807. The physician should discuss the risks and benefits of the use of VISTASEAL with the patient. [see Patient Counseling Information (17)]
-
6 ADVERSE REACTIONS
The most common adverse reactions (reported in > 1% of clinical trial subjects) were nausea and procedural pain.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Three clinical trials were conducted using the same general design, with each being randomized and active controlled. The types of surgeries included in each trial were different. Across all trials, 26% were vascular surgeries, 37% were parenchymous tissue surgeries, and 37% were soft tissue surgeries. The clinical trials were conducted with Fibrin Sealant (Human) using Fibrijet® drip or spray applicators.
Fibrin Sealant (Human) was used to treat vascular bleeding during vascular surgery, or parenchymal bleeding during hepatic surgery, or soft tissue bleeding during retroperitoneal or pelvic surgery, abdominoplasties, or mastopexies, across all clinical trials, involving 500 trial subjects treated with Fibrin Sealant (Human), and 377 control subjects. The number of trial subjects treated with for each type of surgery was 168 for vascular surgery, 163 for parenchymal surgery, and 169 for soft tissue surgery.
The Fibrin Sealant (Human) treatment group had a mean age of 57 years (standard deviation: 15.6 years; range: 0.3 to 86 years). The median age was 60 years. There were 11 subjects younger than 18 years old. There were 51% male subjects. 87% of the subjects exposed to Fibrin Sealant (Human) were White.
The mean volume of Fibrin Sealant (Human) used per subject and target bleeding site was 7 mL (standard deviation 3.5) and ranged from 0.3 to 18 mL. The median volume was 6 mL. Exposure to Fibrin Sealant (Human) consisted of a single intraoperative administration.
The clinical safety database included all subjects who received any amount of Fibrin Sealant (Human) in all clinical studies, with no exclusions.
In the Fibrin Sealant (Human) treatment group, 13% of trial subjects experienced one or more adverse reactions, and 8% of control subjects experienced one or more adverse reactions.
The adverse reactions shown in Tables 3-6 were evaluated as having a possible causal relationship to treatment with Fibrin Sealant (Human) and occurred in >1% of subjects.
Table 3. Adverse Reactions Occurring in >1% of Subjects in Vascular, Parenchyma, and Soft Tissue Surgery Preferred Term N = 500
n (%)Nausea 6 (1) Procedural pain 10 (2) Table 4. Adverse Reactions Occurring in >1% of Subjects in Vascular Surgery Preferred Term N = 168
n (%)Nausea 2 (1) Pyrexia (fever) 2 (1) Procedural pain 4 (2) Vascular graft complication 2 (1) Parvovirus B19 test positive 2 (1) Urinary retention (Unable to empty the bladder completely) 2 (1) Table 5. Adverse Reactions Occurring in >1% of Subjects in Parenchyma Surgery Preferred Term N = 163
n (%)Postprocedural bile leak 2 (1) Procedural pain 2 (1) Pulmonary embolism (Blood clot in the lungs) 2 (1) Deep vein thrombosis (Blood clot that forms in a vein deep) 2 (1) Table 6. Adverse Reactions Occurring in >1% of Subjects in Soft Tissue Surgery Preferred Term N = 169
n (%)Anemia (Low red blood cells) 2 (1) Leukocytosis (Increased white blood cells) 2 (1) Ileus (Decreased or absent movement of the stomach or intestine) 2 (1) Nausea 4 (2) Procedural pain 4 (2) Alanine aminotransferase increased 2 (1) Aspartate aminotransferase increased 2 (1) Hypocalcaemia (Low serum calcium) 2 (1) Hypokalaemia (Low serum potassium) 2 (1) Hyponatraemia (Low serum sodium) 2 (1) Prothrombin time prolonged (Increased bleeding time) 2 (1) Headache 2 (1) Insomnia 2 (1) Pruritus (Itching) 4 (2) Hypertension 2 (1) -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data with VISTASEAL use in pregnant women. Animal reproduction studies have not been performed with VISTASEAL. It is unknown whether VISTASEAL can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. In the U.S. general population, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.8.2 Lactation
Risk Summary
There is no information regarding the presence of VISTASEAL in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VISTASEAL and any potential adverse effects on the breastfed infant from VISTASEAL or from the underlying maternal condition.8.4 Pediatric Use
A total of 11 out of 500 subjects administered Fibrin Sealant (Human) in the clinical trials were pediatric subjects. Of these 11 subjects, 5 were infants aged less than 2 years, 5 were children between the ages of 2 and 11 years, and 1 was an adolescent aged between 12 and 16 years. Safety and effectiveness in pediatric patients have not been established.
-
11 DESCRIPTION
VISTASEAL is a two-component fibrin sealant consisting of human fibrinogen (component 1) and human thrombin with calcium chloride (component 2) sterile solutions filled in syringes which are assembled in a syringe holder.
VISTASEAL is supplied as frozen solutions. After thawing, the human fibrinogen and human thrombin solutions are clear or slightly opalescent and colorless or pale yellow. VISTASEAL does not contain any preservatives.
Fibrinogen
Component 1 is a sterile solution, pH 6.5 – 8.0, which contains concentrated human fibrinogen and excipients. Fibrinogen is a protein from human blood that forms a clot when combined with thrombin. The composition of the human fibrinogen solution is as follows:
Active ingredient: human fibrinogen (80 mg/mL)
Other ingredients: sodium citrate, sodium chloride, arginine, L-isoleucine, L-glutamic acid monosodium and water for injection.
Thrombin
Component 2 is a sterile solution, pH 6.0 – 8.0, which contains purified human thrombin and excipients. Thrombin is a specific protease that activates clotting of the final combined product and converts fibrinogen to fibrin. The composition of the human thrombin solution is as follows:
Active ingredient: human thrombin (500 IU/mL)
Other ingredients: calcium chloride, human albumin, sodium chloride, glycine and water for injection.
The starting material for the production of both fibrinogen and thrombin components of VISTASEAL is pooled human Source Plasma obtained from FDA-licensed plasma collection centers in the United States. Cohn’s plasma fractionation method is used to obtain Fraction I, which is the starting material for the production of fibrinogen, and the prothrombin complex isolated from supernatant of Fraction I, which is the starting material for the production of thrombin. The purification process of fibrinogen includes solvent/detergent treatment, three glycine precipitation steps, and double nanofiltration using 35-nm and 20-nm filters. The purification process of thrombin includes solvent/detergent treatment, ion exchange chromatography, and double nanofiltration through 15-nm filters. After nanofiltration, the fibrinogen and thrombin solutions are formulated, sterile filtered, aseptically filled in syringes, packaged, sterilized, and frozen.
Viral safety
Individual plasma donations used in the manufacture of VISTASEAL are collected in FDA-licensed plasma donation centers in the U.S. and are tested for viral markers in compliance with the U.S. regulatory requirements. In addition, mini-pools of plasma units are tested as an in-process control for hepatitis A virus (HAV) and parvovirus B19 (B19V) using validated nucleic acid testing (NAT) methods. All the tests must be non-reactive (negative) except for B19V, for which the limit in plasma manufacturing pools does not exceed a titer of 104 IU/mL. The manufacturing plasma pool is also tested with NAT for HBV, HCV, and HIV, and all the tests must be non‑reactive (negative).The manufacturing processes for fibrinogen and thrombin include processing steps which are designed to reduce the risk of viral transmission. Both components have two discrete steps with viral clearance capacity, namely solvent/detergent treatment (with 1.0% (v/v) Tween 80/0.30% (v/v) tri-n-butyl phosphate (TNBP) for 6.0 – 6.5 hours at 27.0 ± 1.5 ºC for fibrinogen or 25 ± 1 ºC for thrombin), validated to inactivate enveloped viruses, and a nanofiltration step validated to remove non-enveloped and enveloped viruses (35-nm and 20-nm filters for fibrinogen and two 15-nm filters for thrombin). Additionally, the glycine precipitation steps contribute to the overall safety of the product in the purification process of human fibrinogen. The Fraction I precipitation and ion-exchange chromatography steps contribute to the overall safety of the product in the purification process of human thrombin.
The viral clearance capacity of these virus inactivation/removal procedures has been validated in small-scale in vitro studies using relevant and model viruses with a range of physico-chemical characteristics. The results of viral clearance validation studies are summarized in Tables 7 and 8:
Table 7. Global Virus Reduction Factors (Log10) for Human Fibrinogen Manufacturing step Virus reduction factor (log10)* Enveloped viruses Non-enveloped viruses HIV-1 PRV WNV BVDV HAV PPV S/D treatment ≥ 5.33 ≥ 6.80 ≥ 5.20 ≥ 5.60 n.a. n.a. Glycine
precipitationsn.d. n.d. n.d. n.d. 5.21 2.09 Nanofiltration 35 nm
and 20 nm≥ 5.57 ≥ 6.09 ≥ 4.51 ≥ 4.53 5.22 4.37 Global virus
reduction factor
(log10)≥ 10.90 ≥ 12.89 ≥ 9.71 ≥ 10.13 10.43 6.46 Table 8. Global Virus Reduction Factors (Log10) for Human Thrombin *: Reduction factor below 1 log10 is not considered in calculating the global virus reduction; n.d.: Not done; n.a.: Not applicable; BVDV: bovine viral diarrhea virus, model for HCV; WNV: West Nile virus; PRV: pseudorabies virus, model for large enveloped DNA viruses; PPV: porcine parvovirus, model for B19V Manufacturing step Virus reduction factor (log10)* Enveloped viruses Non-enveloped viruses HIV-1 PRV WNV BVDV HAV PPV Fraction I precipitation < 1.0 2.13 2.78 1.34 1.18 < 1.0 S/D treatment ≥ 5.52 ≥ 5.85 ≥ 5.94 ≥ 5.09 n.a. n.a. SP-Sepharose XL chromatography n.d. n.d. n.d. n.d. 4.61 3.97 Double nanofiltration 15 nm ≥ 4.03 ≥ 5.95 ≥ 5.42 ≥ 4.93 6.56 6.14 Global virus reduction factor (log10) ≥ 9.55 ≥ 13.93 ≥ 14.14 ≥ 11.36 12.35 10.11 -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
VISTASEAL contains human fibrinogen and human thrombin. When applied onto the wound site and mixed, these biological components generate a cross-linked fibrin clot in a process that recreates the last stage of the human blood coagulation system. Fibrinogen is converted into fibrin monomers and fibrinopeptides by thrombin. The fibrin monomers aggregate and form a fibrin clot which stops the bleeding. Factor XIIIa, which is activated from factor XIII by thrombin, crosslinks fibrin. Calcium ions are required for both the conversion of fibrinogen and the crosslinking of fibrin.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
Vascular surgery
A prospective, randomized, controlled clinical study was performed to evaluate the safety and efficacy of Fibrin Sealant (Human) as adjunct to hemostasis in vascular surgery. Subjects underwent vascular surgical procedures utilizing polytetrafluoroethylene graft material on proximal end-to-side arterial anastomosis or upper extremity vascular access arterial anastomosis. The clinical trial was conducted with Fibrin Sealant (Human) using a Fibrijet® applicator. Fibrin Sealant (Human) was shown to be superior to the control group (manual compression) when comparing the proportion of subjects in each group who achieved hemostasis by 4 minutes (Table 9). Superiority was also established at 10 minutes. The median time to hemostasis was significantly shorter (p‑value <0.001) in the Fibrin Sealant (Human) treatment group (4.0 minutes) compared to the control group (≥10.0 minutes).Table 9. Efficacy Results in Vascular Surgery (ITT Population)* *Intent-to-treat (ITT) population: includes all subjects randomized to Fibrin Sealant (Human) or control.
1 The ratio of proportion of subjects meeting the efficacy endpoint in the two treatment groups (Fibrin Sealant (Human) relative to control).
CI = confidence interval.
Tabulated efficacy results are cumulative results.Efficacy endpoints Fibrin Sealant
(Human)
N=109
n (%)Control
N=57
n (%)Ratio of -proportions1
(95% CI)P-value Hemostasis by 4 minutes 83 (76.1) 13 (22.8) 3.3 (2.0, 5.4) <0.001 Hemostasis by 10 minutes 96 (88.1) 26 (45.6) 1.9 (1.4, 2.6) <0.001 Parenchyma surgery
A prospective, randomized, controlled clinical study was performed to evaluate the safety and efficacy of Fibrin Sealant (Human) as adjunct to hemostasis in parenchyma surgery. Subjects underwent liver resections. The clinical trial was conducted with Fibrin Sealant (Human) using a Fibrijet® applicator. Fibrin Sealant (Human) was shown to be superior to the control group (oxidized regenerated cellulose) in achieving hemostasis by 4 minutes (Table 10).
The median time to hemostasis was significantly shorter (p-value <0.001) in the Fibrin Sealant (Human) treatment group (2.0 minutes) compared to the control group (3.0 minutes).Table 10. Efficacy Results in Liver Surgery (ITT Population)* *Intent-to-treat (ITT) population: includes all subjects randomized to Fibrin Sealant (Human) or control.
1 The ratio of proportions of subjects meeting the efficacy endpoint in the two treatment groups (Fibrin Sealant (Human) relative to control).
CI = confidence interval
Tabulated efficacy results are cumulative results.Efficacy endpoints Fibrin Sealant
(Human)
N = 111
n (%)Control
N = 113
n (%)Ratio of proportions 1
(95% CI)P-value Hemostasis by 4 minutes 103 (92.8) 91 (80.5) 1.2 (1.0, 1.3) 0.010 Hemostasis by 2 minutes 62 (55.9) 47 (41.6) 1.3 (1.0, 1.8) 0.045 Soft tissue surgery
A prospective, randomized, controlled clinical study was performed to evaluate the safety and efficacy of Fibrin Sealant (Human) as adjunct to hemostasis in soft tissue bleeding during retroperitoneal and pelvic surgical procedures, and during mastopexies and abdominoplasties. The clinical trial was conducted with Fibrin Sealant (Human) using a Fibrijet® applicator. Fibrin Sealant (Human) was shown to be non-inferior to the control group (oxidized regenerated cellulose) in achieving hemostasis by 4 minutes (Table 11).Table 11. Efficacy results in retroperitoneal and pelvic surgery, mastopexies and abdominoplasties (ITT population)* *Intent-to-treat (ITT) population: includes all subjects randomized to Fibrin Sealant (Human).
1The ratio of proportions of subjects meeting the efficacy endpoint in the two treatment groups (Fibrin Sealant (Human) relative to control).
CI = confidence interval.
Tabulated efficacy results are cumulative results.Efficacy endpoints Fibrin Sealant
(Human)
N = 116
n (%)Control
N = 108
n (%)Ratio of proportions1
(95% CI)P-value Hemostasis by 4 minutes 96 (82.8) 84 (77.8) 1.1 (0.9, 1.2) 0.401 -
16 HOW SUPPLIED/STORAGE AND HANDLING
VISTASEAL is comprised of two pre-filled syringes containing sterile frozen solutions of human fibrinogen (component 1) and human thrombin with calcium chloride (component 2), which are assembled in a syringe holder for single use. The syringe plungers are connected by a plunger link to ensure simultaneous application of the biological components. One Dual Applicator with two additional Airless Spray Tips is co-packaged with the product for application by spraying or dripping. The Airless Spray Tips are radiopaque. See Figure 9.
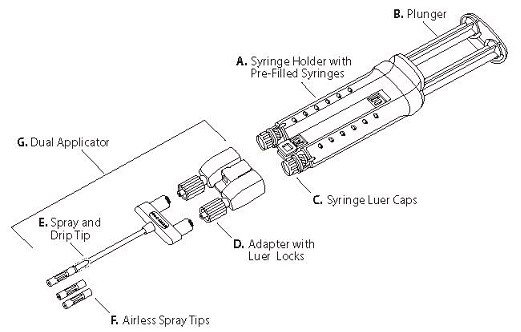
Figure 9
The available package sizes for VISTASEAL are shown in Table 12.
Table 12. VISTASEAL Package Sizes and NDC numbers VISTASEAL Package Size NDC Numbers Total Volume Human
fibrinogenHuman
thrombinCarton Blister label 2 mL 1 mL 1 mL 61953-0011-1 61953-0011-2 4 mL 2 mL 2 mL 61953-0012-1 61953-0012-2 6 mL 3 mL 3 mL 61953-0013-1 61953-0013-2 10 mL 5 mL 5 mL 61953-0014-1 61953-0014-2 Storage
Store the frozen kit (VISTASEAL Fibrin Sealant (Human) with VISTASEAL Dual Applicator) in a freezer (at -18 °C [0 ºF] or colder) for up to 2 years. The cold storage condition must not be interrupted until use. Thaw before use. Once thawed, do not refreeze.After thawing, VISTASEAL can be stored before use for not more than 48 hours at 2 ‑ 8 ºC [36 - 46 ºF] or 24 hours at room temperature (20 - 25 °C [68 - 77 ºF]) if it remains sealed in the original packaging. Once the package is opened, use VISTASEAL immediately during the surgery and discard any unused contents.
Keep the sterilized blister in the outer carton to protect from light.
Do not use after the expiration date printed on the outer carton and container labels. Discard if the package is damaged.
-
17 PATIENT COUNSELING INFORMATION
Instruct patients to immediately report to their physician symptoms of thrombosis or embolism which may include: pain and/or swelling of an arm or leg with warmth over the affected area, discoloration of an arm or leg, unexplained shortness of breath, chest pain or discomfort that worsens on deep breathing, unexplained rapid pulse, numbness or weakness on one side of the body [see Thrombosis (5.1)].
Inform patients that VISTASEAL is made from human plasma and may carry a risk of transmitting infectious agents (e.g., viruses, the vCJD agent and, theoretically, the CJD agent). Instruct patients to report any symptoms that concern them and might be caused by infections.
Manufactured by:
INSTITUTO GRIFOLS, S.A.
BARCELONA - SPAIN
U.S. License No. 1181
Distributed by:
Ethicon, Inc.
1000 Route 202
Raritan, New Jersey 08869
USA • 1-877-ETHICON • +1-513-337-6928
© Ethicon, Inc. 2022
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 61953-0011-1
ETHICON
VistaSeal™
Fibrin Sealant (Human)
VISTASEAL™
Frozen solutions
2 mL
ETHICON
Reorder number
VST02
Fibrin Sealant (Human)
VISTASEAL™
Frozen solutions
2 mL
ETHICON
Fibrin Sealant (Human)
VISTASEAL™
Frozen solutions
2 mL
Human Fibrinogen / Human Thrombin - For topical use. Do not inject.
CONTENTS
• 2 pre-filled syringes, each with a sterile frozen solution, assembled in a syringe holder for
single use.
• 1 Dual Applicator with 2 additional Airless Spray Tips.
Component 1: 1 mL of human fibrinogen (80 mg/mL). Other ingredients: Sodium citrate,
sodium chloride, arginine, L-isoleucine, L-glutamic acid monosodium and water for injection.
Component 2: 1 mL of human thrombin (500 IU/mL). Other ingredients: Calcium chloride,
human albumin, sodium chloride, glycine and water for injection.
Contains no preservatives.
Rx only
NDC 61953-0011-1
Fibrin Sealant (Human)
VISTASEAL™
Frozen solutions
2 mL
Distributed by:
Ethicon, Inc.
1000 Route 202
Raritan, New Jersey 08869
USA • 1-877-ETHICON • +1-513-337-6928
© Ethicon, Inc. 2022
pat. www.ethicon.com/patentmarking
Manufactured by:
Instituto Grifols, S.A.
2 Can Guasch St. Polígono Levante
08150 Parets del Vallès
Barcelona - SPAIN
U.S. License No. 1181
Store in a freezer (-18 °C [0 °F] or colder). The cold storage chain must not be interrupted until use.
Keep the sterilized blister in the outer carton in order to protect from light.
Thaw for use. Do not refreeze once thawed. After thawing, the kit can be stored before use for not
more than 48 hours at 2 - 8 °C [36 - 46 °F] or 24 hours at room temperature (20 - 25 °C [68 - 77 °F]) if
it remains sealed in the original packaging. Once packaging is opened, use product immediately.
For usage instructions and precautions, see enclosed package insert.
Discard any unused contents and administration devices after use.
Reorder number
VST02
Fibrin Sealant (Human)
VISTASEAL™
Frozen solutions
2 mL
ETHICON
GTIN: 00361953001118
SN: XXXXXXXXXXXXXXXX
Lot XXXXXXXXXX
EXP DD-MMM-YYYY
3063053
VistaSeal™
Fibrinogen
80 mg/mL
1 mL
Lot
EXP
3060674

VistaSeal™
Thrombin
500 IU/mL
1 mL
Lot
EXP
3060675

VistaSeal™ 2mL
Lot
EXP
Thawed Exp:
3055495
Fibrin Sealant (Human)
VistaSeal™
Frozen solutions
NDC 61953-0011-2
2 mL
Rx only
Human Fibrinogen / Human Thrombin - For topical use. Do not inject.
CONTENTS
2 pre-filled syringes, each with a sterile frozen solution, assembled in a syringe holder for single use.
Component 1: 1 mL of human fibrinogen (80 mg/mL). Other ingredients: Sodium citrate, sodium chloride,
arginine, L-isoleucine, L-glutamic acid monosodium and water for injection.
Component 2: 1 mL of human thrombin (500 IU/mL). Other ingredients: Calcium chloride,
human albumin, sodium chloride, glycine and water for injection.
Contains no preservatives. Store in a freezer (-18 °C [0 °F] or colder). Thaw for use.
Do not refreeze once thawed. For usage instructions and precautions, see enclosed package insert.
ETHICON
LOT
EXP
Manufactured by: Instituto Grifols, S.A.
Barcelona - SPAIN. U.S. License No. 1181
3063058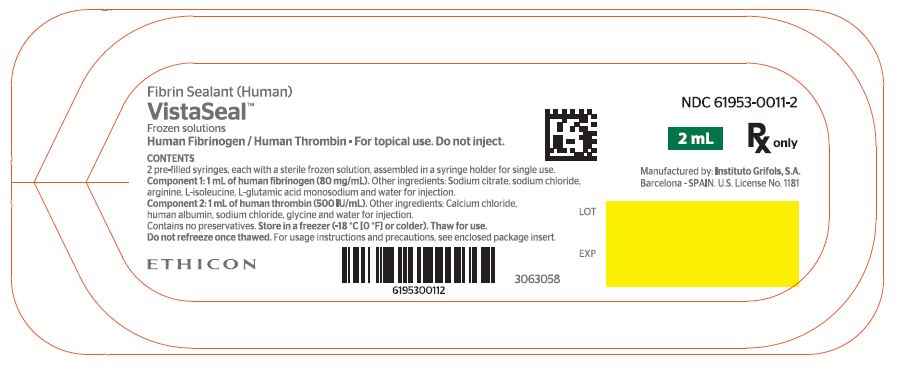
-
INGREDIENTS AND APPEARANCE
VISTASEAL
human fibrinogen, human thrombin kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:61953-0011 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61953-0011-1 1 in 1 PACKAGE, COMBINATION; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE, GLASS 1 mL Part 2 1 SYRINGE, GLASS 1 mL Part 1 of 2 FIBRINOGEN
fibrinogen solutionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fibrinogen Human (UNII: N94833051K) (Fibrinogen Human - UNII:N94833051K) Fibrinogen Human 80 mg in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Citrate, Unspecified Form (UNII: 1Q73Q2JULR) Sodium Chloride (UNII: 451W47IQ8X) Arginine (UNII: 94ZLA3W45F) Isoleucine (UNII: 04Y7590D77) Monosodium Glutamate Anhydrous (UNII: C3C196L9FG) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125640 11/01/2019 Part 2 of 2 HUMAN THROMBIN
human thrombin solutionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Thrombin Human (UNII: 6K15ABL77G) (Thrombin Human - UNII:6K15ABL77G) Thrombin Human 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength Calcium Chloride (UNII: M4I0D6VV5M) Albumin Human (UNII: ZIF514RVZR) Sodium Chloride (UNII: 451W47IQ8X) Glycine (UNII: TE7660XO1C) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125640 11/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125640 11/01/2019 VISTASEAL
human fibrinogen, human thrombin kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:61953-0012 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61953-0012-1 1 in 1 PACKAGE, COMBINATION; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE, GLASS 2 mL Part 2 1 SYRINGE, GLASS 2 mL Part 1 of 2 FIBRINOGEN
fibrinogen solutionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fibrinogen Human (UNII: N94833051K) (Fibrinogen Human - UNII:N94833051K) Fibrinogen Human 80 mg in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Citrate, Unspecified Form (UNII: 1Q73Q2JULR) Sodium Chloride (UNII: 451W47IQ8X) Arginine (UNII: 94ZLA3W45F) Isoleucine (UNII: 04Y7590D77) Monosodium Glutamate Anhydrous (UNII: C3C196L9FG) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125640 11/01/2019 Part 2 of 2 HUMAN THROMBIN
human thrombin solutionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Thrombin Human (UNII: 6K15ABL77G) (Thrombin Human - UNII:6K15ABL77G) Thrombin Human 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength Calcium Chloride (UNII: M4I0D6VV5M) Albumin Human (UNII: ZIF514RVZR) Sodium Chloride (UNII: 451W47IQ8X) Glycine (UNII: TE7660XO1C) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125640 11/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125640 11/01/2019 VISTASEAL
human fibrinogen, human thrombin kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:61953-0013 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61953-0013-1 1 in 1 PACKAGE, COMBINATION; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE, GLASS 3 mL Part 2 1 SYRINGE, GLASS 3 mL Part 1 of 2 FIBRINOGEN
fibrinogen solutionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fibrinogen Human (UNII: N94833051K) (Fibrinogen Human - UNII:N94833051K) Fibrinogen Human 80 mg in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Citrate, Unspecified Form (UNII: 1Q73Q2JULR) Sodium Chloride (UNII: 451W47IQ8X) Arginine (UNII: 94ZLA3W45F) Isoleucine (UNII: 04Y7590D77) Monosodium Glutamate Anhydrous (UNII: C3C196L9FG) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125640 11/01/2019 Part 2 of 2 HUMAN THROMBIN
human thrombin solutionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Thrombin Human (UNII: 6K15ABL77G) (Thrombin Human - UNII:6K15ABL77G) Thrombin Human 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength Calcium Chloride (UNII: M4I0D6VV5M) Albumin Human (UNII: ZIF514RVZR) Sodium Chloride (UNII: 451W47IQ8X) Glycine (UNII: TE7660XO1C) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125640 11/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125640 11/01/2019 VISTASEAL
human fibrinogen, human thrombin kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC:61953-0014 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61953-0014-1 1 in 1 PACKAGE, COMBINATION; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE, GLASS 5 mL Part 2 1 SYRINGE, GLASS 5 mL Part 1 of 2 FIBRINOGEN
fibrinogen solutionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fibrinogen Human (UNII: N94833051K) (Fibrinogen Human - UNII:N94833051K) Fibrinogen Human 80 mg in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Citrate, Unspecified Form (UNII: 1Q73Q2JULR) Sodium Chloride (UNII: 451W47IQ8X) Arginine (UNII: 94ZLA3W45F) Isoleucine (UNII: 04Y7590D77) Monosodium Glutamate Anhydrous (UNII: C3C196L9FG) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 5 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125640 11/01/2019 Part 2 of 2 HUMAN THROMBIN
human thrombin solutionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Thrombin Human (UNII: 6K15ABL77G) (Thrombin Human - UNII:6K15ABL77G) Thrombin Human 500 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength Calcium Chloride (UNII: M4I0D6VV5M) Albumin Human (UNII: ZIF514RVZR) Sodium Chloride (UNII: 451W47IQ8X) Glycine (UNII: TE7660XO1C) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 5 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125640 11/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125640 11/01/2019 Labeler - GRIFOLS USA, LLC (048987452) Establishment Name Address ID/FEI Business Operations Instituto Grifols SA 465562213 manufacture(61953-0011, 61953-0012, 61953-0013, 61953-0014)