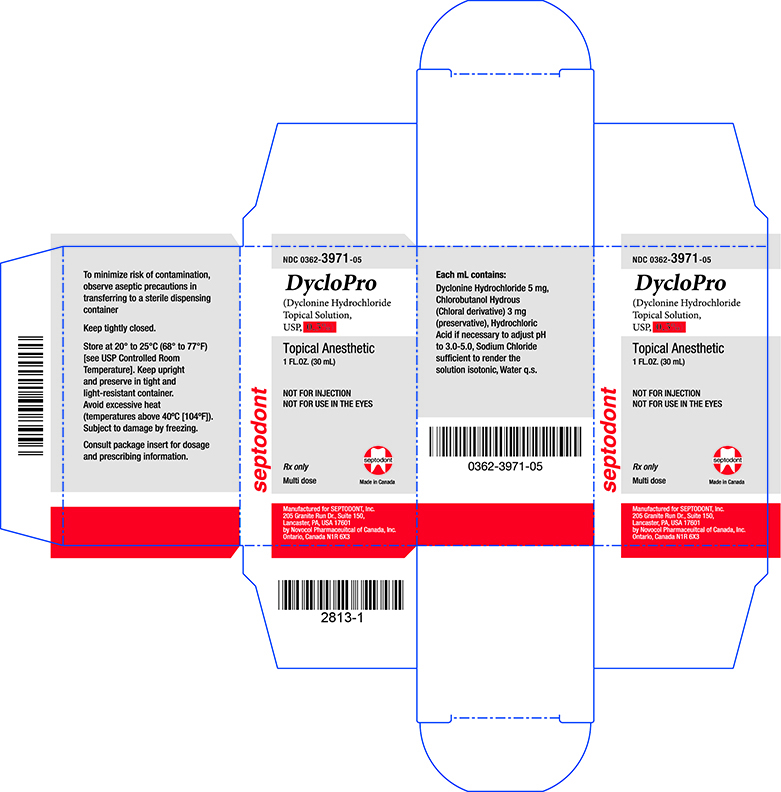
Label: DYCLOPRO- dyclonine hydrochloride solution
- NDC Code(s): 0362-3971-05
- Packager: Septodont Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 6, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Description:
DycloPro (Dyclonine Hydrochloride Topical Solution, USP, 0.5% and 1%) topical anesthetics contain a local anesthetic agent and are administered topically. See INDICATIONS for specific uses.
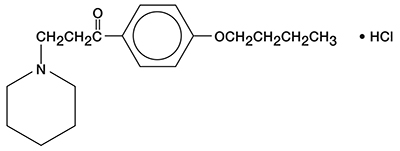
DycloPro (Dyclonine Hydrochloride Topical Solution, USP, 0.5% and 1%) contain dyclonine HCl, which is chemically designated as 4’-butoxy-3-piperidinopropiophenone HCl. Dyclonine HCl is a white crystalline powder that is sparingly soluble in water and has the following structural formula:
- COMPOSITION OF Dyclonine HCl Topical Solution, USP, 0.5% and 1% topical anesthetics:
- CLINICAL PHARMACOLOGY:
-
INDICATIONS AND USAGE:
Dyclonine HCl Topical Solution, USP is indicated for anesthetizing accessible mucous membranes (e.g., the mouth, pharynx, larynx, trachea, esophagus, and urethra) prior to various endoscopic procedures.
Dyclonine HCl Topical Solution, USP, 0.5% topical anesthetic may also be used to block the gag reflex, to relieve the pain of oral ulcers or stomatitis and to relieve pain associated with ano-genital lesions.
- CONTRAINDICATIONS:
-
WARNINGS:
IN ORDER TO MANAGE POSSIBLE ADVERSE REACTIONS, RESUSCITATIVE EQUIPMENT, OXYGEN AND OTHER RESUSCITATIVE DRUGS SHOULD BE IMMEDIATELY AVAILABLE WHENEVER LOCAL ANESTHETIC AGENTS, SUCH AS DYCLONINE, ARE ADMINISTERED TO MUCOUS MEMBRANES.
Dyclonine HCl Topical Solution, USP should not be injected into tissue or used in the eyes because of highly irritant properties.
Dyclonine HCl Topical Solution, USP should be used with extreme caution in the presence of sepsis or severely traumatized mucosa in the area of application since under such conditions there is the potential for rapid systemic absorption.
-
PRECAUTIONS:
General: The safety and effectiveness of dyclonine depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies (see WARNINGS and ADVERSE REACTIONS). The lowest dosage that results in effective anesthesia should be used to avoid high plasma levels and serious adverse effects. Repeated doses of dyclonine may cause significant increases in blood levels with each repeated dose because of slow accumulation of the drug or its metabolites. Tolerance to elevated blood levels varies with the status of the patient. Debilitated, elderly patients, acutely ill patients and children should be given reduced doses commensurate with their age, weight and physical condition. Dyclonine should also be used with caution in patients with severe shock or heart block.
Dyclonine HCl Topical Solution, USP should be used with caution in persons with known drug sensitivities.
-
Information for Patients:
When topical anesthetics are used in the mouth or throat, the patient should be aware that the production of topical anesthesia may impair swallowing and thus enhance the danger of aspiration. For this reason, food should not be ingested for 60 minutes following use of local anesthetic preparations in the mouth or throat area. This is particularly important in children because of their frequency of eating.
Numbness of the tongue or buccal mucosa may increase the danger of biting trauma. When Dyclonine HCl Topical Solution, USP is used to relieve the pain of oral ulcers or stomatitis which interferes with eating, patients should be warned about the risk of biting trauma before they accept this treatment; caution should be exercised in selecting food and eating. Following other uses in the mouth and throat area, food and/or chewing gum should not be used while the area is anesthetized.
- Drug/Laboratory Test Interactions:
- Carcinogenesis, mutagenesis, impairment of fertility:
-
Pregnancy:Teratogenic Effects:
Pregnancy Category C. Animal reproduction studies have not been conducted with dyclonine hydrochloride. It is also not known whether dyclonine hydrochloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Dyclonine hydrochloride should be given to pregnant women only if clearly needed.
- Nursing Mothers:
- Pediatric Use:
-
ADVERSE REACTIONS:
Adverse experiences following the administration of dyclonine are similar in nature to those observed with other local anesthetic agents. These adverse experiences are, in general, dose-related and may result from high plasma levels caused by excessive dosage or rapid absorption, or may result from a hypersensitivity, idiosyncrasy or diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature. The following types are those most commonly reported:
Central nervous system:
CNS manifestations are excitatory and/or depressant and may be characterized by lightheadedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest. The excitatory manifestations may be very brief or may not occur at all, in which case the first manifestation of toxicity may be drowsiness merging into unconsciousness and respiratory arrest.
Drowsiness following the administration of dyclonine is usually an early sign of a high blood level of the drug and may occur as a consequence of rapid absorption.
Cardiovascular system:
Cardiovascular manifestations are usually depressant and are characterized by bradycardia, hypotension, and cardiovascular collapse, which may lead to cardiac arrest.
Allergic:
Allergic reactions are characterized by cutaneous lesions, urticaria, edema or anaphylactoid reactions. Allergic reactions may occur as a result of sensitivity either to the local anesthetic agent or to the other ingredients used in this formulation. Allergic reactions, if they occur, should be managed by conventional means. The detection of sensitivity by skin testing is of doubtful value. Local reactions include irritation, stinging, urethritis with and without bleeding.
-
OVERDOSAGE:
Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics. (See ADVERSE REACTIONS, WARNINGS and PRECAUTIONS).
Management of local anesthetic emergencies: The first consideration is prevention, best accomplished by careful and constant monitoring of cardiovascular and respiratory vital signs and the patient’s state of consciousness after each local anesthetic administration.
The first step in the management of convulsions consists of immediate attention to the maintenance of a patent airway and assisted or controlled ventilation with oxygen and a delivery system capable of permitting immediate positive airway pressure by mask. Immediately after the institution of these ventilatory measures, the adequacy of the circulation should be evaluated, keeping in mind that drugs used to treat convulsions sometimes depress the circulation when administered intravenously. Should convulsions persist despite adequate respiratory support, and if the status of the circulation permits, small increments of an ultra-short acting barbiturate (such as thiopental or thiamylal) or a benzodiazepine (such as diazepam) may be administered intravenously. The clinician should be familiar, prior to use of local anesthetics, with these anticonvulsant drugs. Supportive treatment of circulatory depression may require administration of intravenous fluids and, when appropriate, a vasopressor as directed by the clinical situation (e.g., ephedrine).
If not treated immediately, both convulsions and cardiovascular depression can result in hypoxia, acidosis, bradycardia, arrhythmias and cardiac arrest. If cardiac arrest should occur, standard cardiopulmonary resuscitative measures should be instituted.
The median lethal dose (LD50) of Dyclonine HCl Topical Solution, USP administered orally to female rats is 176 mg/kg and 90 mg/kg in female mice. Intraperitoneally the LD50 in female rats is 31 mg/kg and 43 mg/kg in female mice.
-
DOSAGE AND ADMINISTRATION:
As with all local anesthetics, the dosage varies and depends upon the area to be anesthetized, vascularity of the tissues, individual tolerance and the technique of anesthesia. The lowest dosage needed to provide effective anesthesia should be administered.
A maximum dose of 30 mL of Dyclonine HCl Topical Solution, USP 1% topical anesthetic (300 mg of dyclonine HCl) may be used, although satisfactory anesthesia is usually produced within the range of 4 to 20 mL. For specific techniques and procedures refer to standard textbooks.
Although as much as 300 mg of Dyclonine HCl Topical Solution USP (as a 1% solution) have been tolerated, this dosage as a 0.5% solution has not been administered primarily because satisfactory anesthesia in endoscopic procedures can usually be produced by lesser amounts. For specific techniques for endoscopic procedures refer to standard textbooks.
Dyclonine HCl Topical Solution, USP can be used for a period of 7 days after open.
- PROCTOLOGY:
- GYNECOLOGY:
-
ONCOLOGY-RADIOLOGY:
Apply Dyclonine HCl Topical Solution, USP 0.5% as a rinse or swab to inflamed or ulcerated mucous membrane of the mouth caused by anti-neoplastic chemotherapy or radiation therapy. In lesions of the esophagus, 5-15 mL of the anesthetic may be swallowed to relieve pain and allow more comfortable deglutition.
-
OTORHINOLARYNGOLOGY:
To suppress the gag reflex and to facilitate examination of the posterior pharynx or larynx, apply Dyclonine HCl Topical Solution, USP, 0.5% as a spray or gargle.
Dyclonine HCl Topical Solution, USP, 0.5% may be applied as a rinse or swab to relieve the discomfort of aphthous stomatitis, herpetic stomatitis, or other painful oral lesions.
-
DENTISTRY:
Dyclonine HCl Topical Solution, USP, 0.5% topical anesthetic is useful to suppress the gag reflex in the positioning of x-ray films, making prosthetic impressions, and doing surgical procedures in the molar areas. It is also useful as a preinjection mucous membrane anesthetic or applied to the gums prior to scaling (prophylaxis). The anesthetic can be applied as a mouthwash or gargle and the excess spit out.
-
HOW SUPPLIED:
Clear and colorless sterile solution, in one fluid ounce bottles.
Multi dose
Dyclonine HCl Topical Solution 0.5% (NDC 0362-3971-05)
Dyclonine HCl Topical Solution 1% (NDC 0362-3918-10).
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Keep upright and preserve in tight and light-resistant container. Avoid excessive heat (temperatures above 40ºC [104ºF]). Subject to damage by freezing. - SPL UNCLASSIFIED SECTION
-
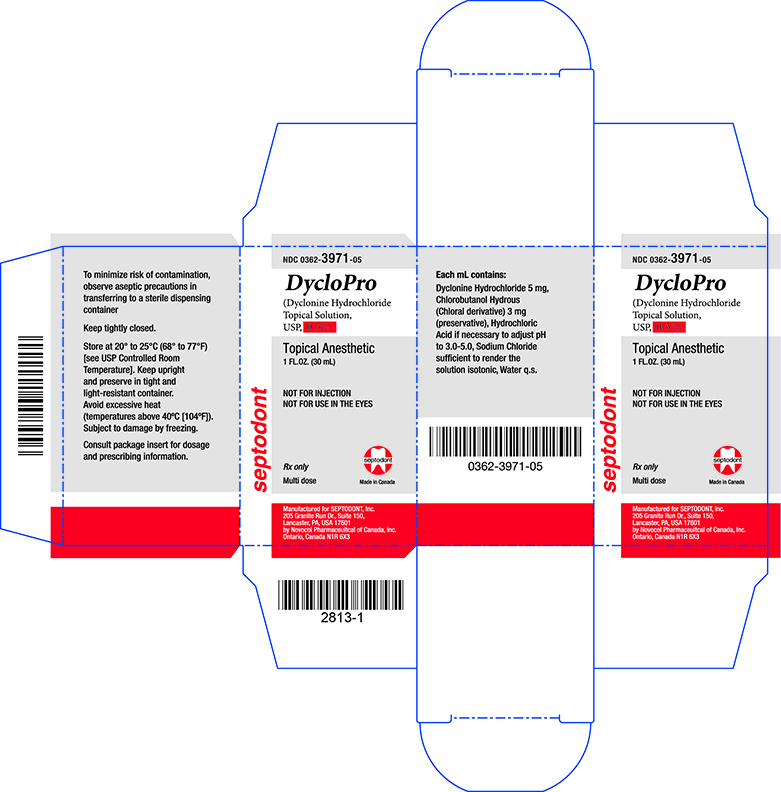
Principal Display Panel - Carton - 0.5%
NDC 0362-3971-05
DycloPro
(Dyclonine Hydrochloride Topical Solution, USP, 0.5%)
Topical Anesthetic
1FL.OZ. (30mL)
NOT FOR INJECTION
NOT FOR USE IN THE EYES.
Rx only
Multi dose
Manufactured for SEPTODONT, Inc.
205 Granite Run Dr., Suite 150,
Lancaster, PA, USA 17601
by Novocol Pharmaceutical of Canada, Inc.
Ontario, Canada N1R 6X3
-
INGREDIENTS AND APPEARANCE
DYCLOPRO
dyclonine hydrochloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0362-3971 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DYCLONINE HYDROCHLORIDE (UNII: ZEC193879Q) (DYCLONINE - UNII:078A24Q30O) DYCLONINE HYDROCHLORIDE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength CHLOROBUTANOL (UNII: HM4YQM8WRC) 3 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0362-3971-05 1 in 1 BOTTLE 09/01/2019 1 30 mL in 1 BOTTLE; Number of Units = 1; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200480 09/01/2019 Labeler - Septodont Inc (627058738) Establishment Name Address ID/FEI Business Operations Novocol Pharmaceutical of Canada, Inc. 201719960 manufacture(0362-3971)