Label: ZERVIATE- cetirizine solution/ drops
- NDC Code(s): 71776-024-01, 71776-024-05, 71776-024-30
- Packager: Eyevance Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
ZERVIATE™ (cetirizine ophthalmic solution) 0.24%, for topical ophthalmic use
These highlights do not include all the information needed to use ZERVIATE safely and effectively. See full prescribing information for ZERVIATE. Initial U.S. Approval: 1995RECENT MAJOR CHANGES
Dosage and Administration (2) 2/2020
Warnings and Precautions (5.1) 2/2020
INDICATIONS AND USAGE
ZERVIATE (cetirizine ophthalmic solution) 0.24% is a histamine-1 (H1) receptor antagonist indicated for treatment of ocular itching associated with allergic conjunctivitis. ( 1)
DOSAGE AND ADMINISTRATION
The recommended dose is one drop in each affected eye twice daily. ( 2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution: 2.4 mg cetirizine in 1 mL sterile solution (0.24%). ( 3)
CONTRAINDICATIONS
None. ( 4)
WARNINGS AND PRECAUTIONS
Contamination of Tip and Solution. To prevent contaminating the dropper tip and solution, advise patients not to touch the eyelids or surrounding areas with the dropper tip of the bottle or tip of single-use container. ( 5.1)
ADVERSE REACTIONS
The most common adverse reactions (1–7%) were ocular hyperemia, instillation site pain, and visual acuity reduced. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Eyevance Pharmaceuticals, LLC at 1-844-390-0720 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
RECENT MAJOR CHANGES
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Contamination of Tip and Solution
5.2 Contact Lens Wear
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
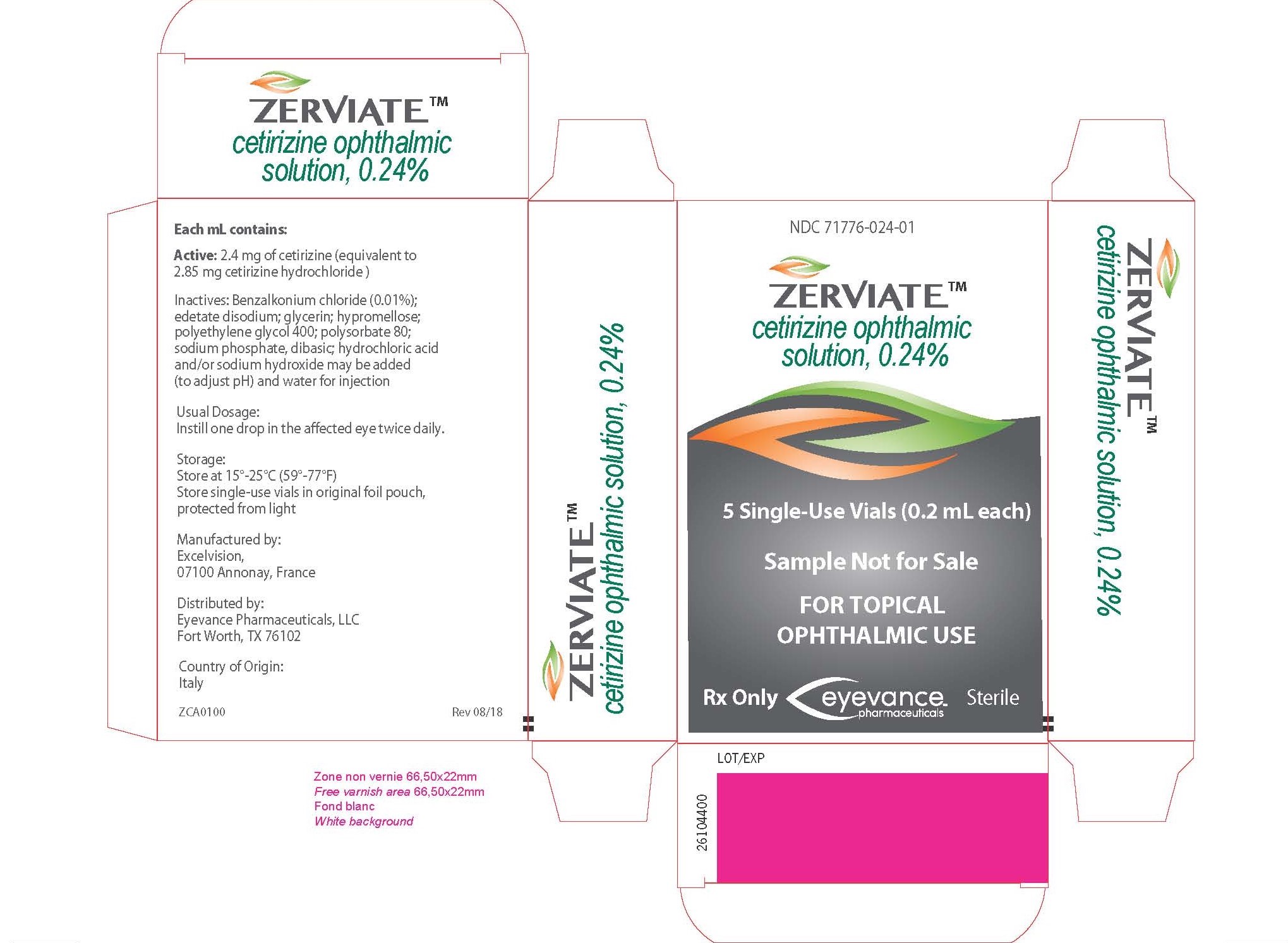
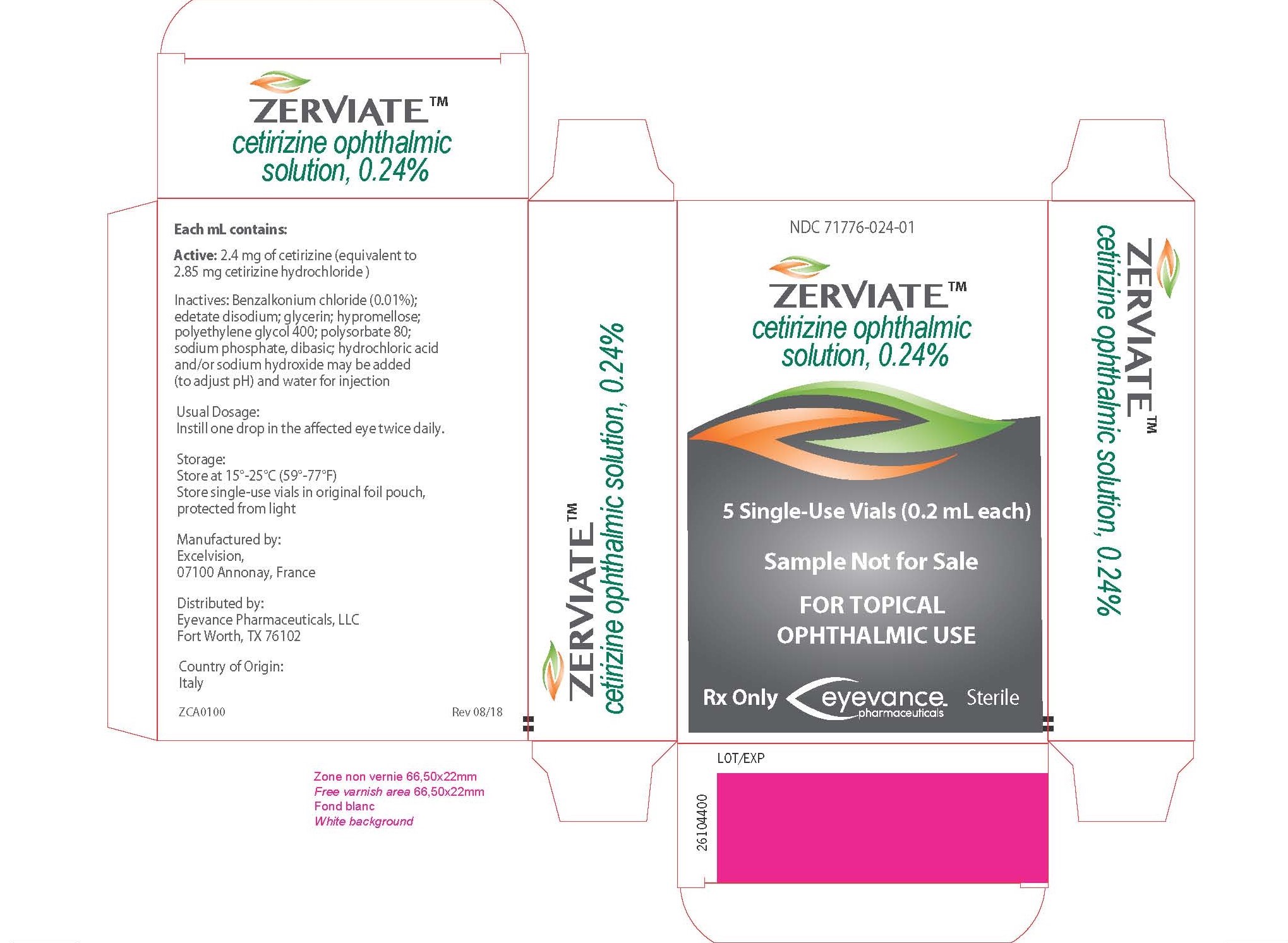
The recommended dosage of ZERVIATE is to instill one drop in each affected eye twice daily (approximately 8 hours apart).
│The single-use containers are to be used immediately after opening and can be used to dose both eyes. Discard the single-use container and any remaining contents after administration. The single-use │containers should be stored in the original foil pouch until ready to use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Contamination of Tip and Solution
│As with any eye drop, care should be taken not to touch the eyelids or surrounding areas with the dropper tip of the bottle or tip of the single- use container in order to avoid injury to the eye and to prevent │contaminating the tip and solution. Keep the multi-dose bottle closed when not in use. Discard the single-use container after using in each eye.
5.2 Contact Lens Wear
Patients should be advised not to wear a contact lens if their eye is red.
ZERVIATE should not be instilled while wearing contact lenses. Remove contact lenses prior to instillation of ZERVIATE. The preservative in ZERVIATE, benzalkonium chloride, may be absorbed by soft contact lenses. Lenses may be reinserted 10 minutes following administration of ZERVIATE.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trial of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates in practice.
In seven clinical trials, patients with allergic conjunctivitis or those at a risk of developing allergic conjunctivitis received one drop of either cetirizine (N=511) or vehicle (N=329) in one or both eyes. The most commonly reported adverse reactions occurred in approximately 1–7% of patients treated with either ZERVIATE or vehicle. These reactions were ocular hyperemia, instillation site pain, and visual acuity reduced.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
Risk Summary
Cetirizine has been reported to be excreted in human breast milk following oral administration. Multiple doses of oral dose cetirizine (10 mg tablets once daily for 10 days) resulted in systemic levels (Mean C max = 311 ng/mL) that were 100 times higher than the observed human exposure (Mean C max = 3.1 ng/mL) following twice-daily administration of cetirizine ophthalmic solution 0.24% to both eyes for one week [see Clinical Pharmacology ( 12.3)] . Comparable bioavailability has been found between the tablet and syrup dosage forms. However, it is not known whether the systemic absorption resulting from topical ocular administration of ZERVIATE could produce detectable quantities in human breast milk.
There is no adequate information regarding the effects of cetirizine on breastfed infants, or the effects on milk production to inform risk of ZERVIATE to an infant during lactation. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ZERVIATE and any potential adverse effects on the breastfed child from ZERVIATE.
-
11 DESCRIPTION
ZERVIATE is a sterile opthalmic solution containing cetirizene, which is a histamine-1 (H1) receptor antagonist, for topical administration to the eyes. Cetirizine hydrochloride is a white, crystalline, water-soluble powder with a molecular weight of 461.8 and a molecular formula of C 21H 25ClN 20 3•2HCl. The chemical structure is presented below:
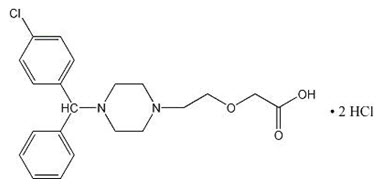
Chemical Name: ( RS)-2-[2-[4-[(4-Chlorophenyl) phenylmethyl] piperazin-1-yl] ethoxy] acetic acid, dihydrochloride
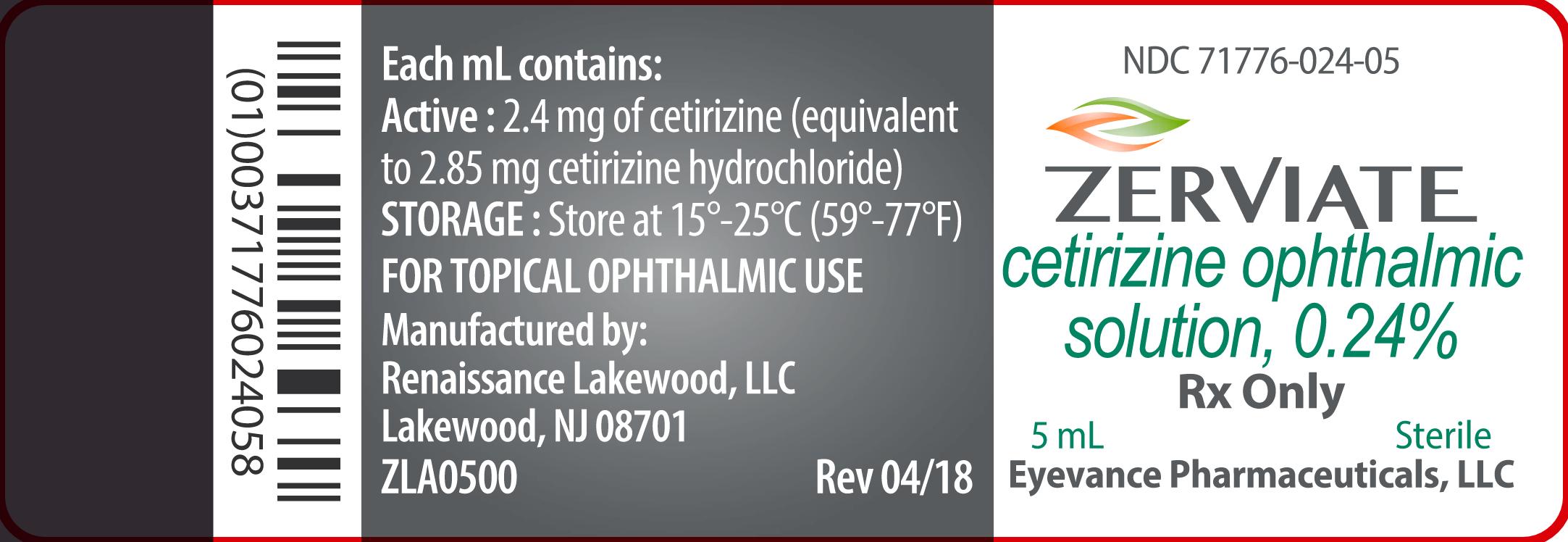
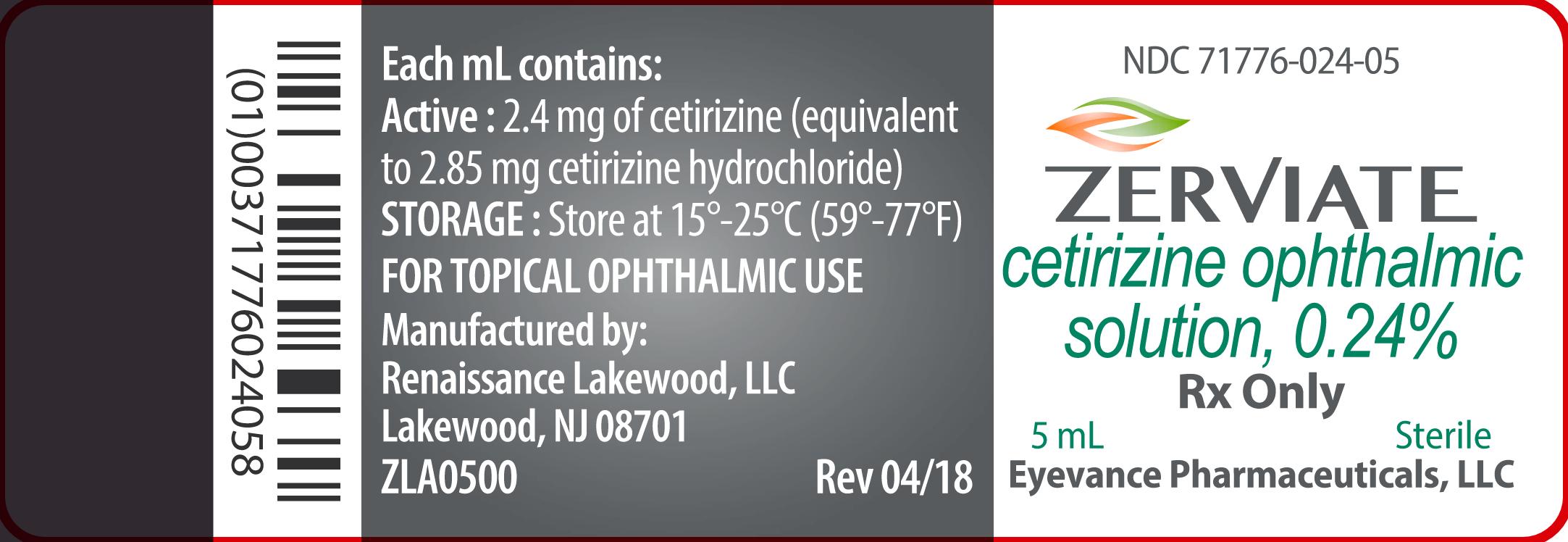
Each mL of ZERVIATE contains an active ingredient [cetirizine 2.40 mg (equivalent to 2.85 mg of cetirizine hydrochloride)] and the following inactive ingredients: benzalkonium chloride 0.010% (preservative); glycerin; sodium phosphate, dibasic; edetate disodium; polyethylene glycol 400; polysorbate 80; hypromellose; hydrochloric acid/sodium hydroxide (to adjust pH); and water for injection. ZERVIATE solution has a pH of approximately 7.0 and osmolality of approximately 300 mOsm/kg.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ZERVIATE, an antihistamine, is a histamine-1 (H1) receptor antagonist. The antihistaminic activity of cetirizine has been documented in a variety of animal and human models. In vivo and ex vivo animal models have shown negligible anticholinergic and antiserotonergic activity. In vitro receptor binding studies have shown no measurable affinity for other than H1 receptors.
12.3 Pharmacokinetics
In healthy subjects, bilateral topical ocular dosing of one drop of ZERVIATE™ (cetirizine ophthalmic solution) 0.24% resulted in a mean cetirizine plasma C max of 1.7 ng/mL following a single dose and 3.1 ng/mL after twice-daily dosing for one week. The observed mean terminal half-life of cetirizine was 8.6 hours following a single dose and 8.2 hours after twice-daily dosing of ZERVIATE for one week.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
In a 2-year carcinogenicity study in rats, orally administered cetirizine was not carcinogenic at dietary doses up to 20 mg/kg (approximately 550 times the MRHOD, on a mg/m 2 basis). In a 2-year carcinogenicity study in mice, cetirizine caused an increased incidence of benign liver tumors in males at a dietary dose of 16 mg/kg (approximately 220 times the MRHOD, on a mg/m 2 basis). No increase in the incidence of liver tumors was observed in mice at a dietary dose of 4 mg/kg (approximately 55 times the MRHOD, on a mg/m 2 basis). The clinical significance of these findings during long-term use of cetirizine is not known.
Mutagenesis
Cetirizine was not mutagenic in the Ames test or in an in vivo micronucleus test in rats. Cetirizine was not clastogenic in the human lymphocyte assay or the mouse lymphoma assay.
Impairment of Fertility
In a fertility and general reproductive performance study in mice, cetirizine did not impair fertility at an oral dose of 64 mg/kg (approximately 875 times the MRHOD on a mg/m 2 basis).
-
14 CLINICAL STUDIES
The efficacy of ZERVIATE was established in three randomized, double-masked, placebo-controlled, conjunctival allergen challenge (CAC) clinical trials in patients with a history of allergic conjunctivitis.
Onset and duration of action were evaluated in two of these trials in which patients were randomized to receive ZERVIATE or vehicle ophthalmic solutions. Patients were evaluated with an ocular itching severity score ranging from 0 (no itching) to 4 (incapacitating itch) at several time points after CAC administration. Table 1 displays data from the mean ocular itching severity scores after ocular administration of an antigen using the CAC model. A one unit difference compared to vehicle is considered a clinically meaningful change in the ocular itching severity score.
Patients treated with ZERVIATE demonstrated statistically and clinically significantly less ocular itching compared to vehicle at 15 minutes and 8 hours after treatment.
Table 1 Itching Scores in the ITT Population by Treatment Group and Treatment Difference Study 1 Study 2 Statistics 15 minutes post-treatment 8 hours post-treatment 15 minutes post-treatment 8 hours post-treatment ZERVIATE
N=50Vehicle
N=50ZERVIATE
N=50Vehicle
N=50ZERVIATE
N=51Vehicle
N=50ZERVIATE
N=51Vehicle
N=501 Treatment difference values shown are the group mean active minus the group mean vehicle at each post-CAC time point.
* p<0.053 Minute Post-CAC
Mean
1.00
2.38
1.76
2.69
1.01
2.54
1.94
2.86
Treatment Difference
(95% CI) 1-1.38 (-1.72, -1.05)*
-0.93 (-1.26, -0.61)*
-1.53 (-1.92, -1.15)*
-0.92 (-1.25, -0.58)*
5 Minute Post-CAC
Mean
1.18
2.43
1.85
2.74
1.17
2.51
2.03
2.94
Treatment Difference
(95% CI) 1-1.25 (-1.58, -0.91)*
-0.89 (-1.24, -0.54)*
-1.34 (-1.71, -0.97)*
-0.90 (-1.23, -0.57)*
7 Minute Post-CAC
Mean
1.11
2.11
1.54
2.53
1.15
2.23
1.82
2.66
Treatment Difference
(95% CI) 1-1.00 (-1.35, -0.65)*
-0.99 (-1.40, -0.59)*
-1.07 (-1.46, -0.69)*
-0.84 (-1.21, -0.48)*
-
16 HOW SUPPLIED/STORAGE AND HANDLING
ZERVIATE is a sterile, buffered, clear, colorless aqueous solution containing cetirizine 0.24% (equivalent to cetirizine hydrochloride 0.29%) supplied in a white low-density polyethylene multi-dose ophthalmic bottle with a low-density polyethylene dropper tip and a white polypropylene cap. ZERVIATE is supplied in a 7.5 mL bottle that contains 5 mL and a 10 mL bottle that contains 7.5 mL cetirizine ophthalmic solution, 2.40 mg [equivalent to 2.85 mg cetirizine hydrochloride in one mL solution]. ZERVIATE is also supplied in 5 low-density polyethylene 0.2 mL single-use containers within a foil pouch.
5 mL fill in a 7.5 mL bottle NDC 71776-024-05
7.5 mL fill in a 10 mL bottle NDC 71776-024-08
Carton of 30 single-use containers NDC 71776-024-30
Storage: Store at 15°C to 25°C (59°F to 77°F). Single-use containers should be stored in the original foil pouch.
-
17 PATIENT COUNSELING INFORMATION
- Risk of Contamination: Advise patients not to touch dropper tip to eyelids or surrounding areas, as this may contaminate the dropper tip and ophthalmic solution. Advise patients to keep the bottle closed when not in use. Advise patients to discard single-use containers after each use.
- Concomitant Use of Contact Lenses: Advise patients not to wear contact lenses if their eyes are red. Advise patients that ZERVIATE should not be used to treat contact lens-related irritation. Advise patients to remove contact lenses prior to instillation of ZERVIATE. The preservative in ZERVIATE solution, benzalkonium chloride, may be absorbed by soft contact lenses. Lenses may be reinserted ten minutes following administration of ZERVIATE.
- Administration: Advise patients that the solution from one single-use container is to be used immediately after opening. Advise patients that the single-use container can be used to dose both eyes. Discard the single-use container and remaining contents immediately after administration.
- Storage of Single-use Containers: Instruct patients to store single-use containers in the original foil pouch until ready to use.
ZPI0000 Rev 02/2020
Distributed by:
Eyevance Pharmaceuticals, LLC.Fort Worth, TX 76102
U.S. Patents: 8,829,005; 9,254,286; 9,750,684; 9,993,471
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ZERVIATE
cetirizine solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71776-024 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE (UNII: YO7261ME24) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE 2.4 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) HYPROMELLOSE 2910 (4000 MPA.S) (UNII: RN3152OP35) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71776-024-05 1 in 1 CARTON 02/19/2019 1 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC:71776-024-01 5 in 1 CARTON 03/23/2020 2 0.2 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 3 NDC:71776-024-30 30 in 1 CARTON 03/23/2020 3 0.2 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208694 02/19/2019 Labeler - Eyevance Pharmaceuticals (080876046) Registrant - Eyevance Pharmaceuticals (080876046) Establishment Name Address ID/FEI Business Operations Excelvision 274234566 manufacture(71776-024) , analysis(71776-024) , label(71776-024) , pack(71776-024) Establishment Name Address ID/FEI Business Operations COSMA Spa 428655732 api manufacture(71776-024)