Label: CLAVACILLIN- amoxicillin anhydrous and clavulanate potassium tablet
-
NDC Code(s):
17033-440-07,
17033-440-21,
17033-441-07,
17033-441-21, view more17033-442-07, 17033-442-21, 17033-443-07, 17033-443-21
- Packager: Dechra Veterinary Products LLC
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated March 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- SAFE HANDLING WARNING
-
DESCRIPTION
DESCRIPTION:
Clavacillin (amoxicillin and clavulanate potassium tablets), USP is an orally administered formulation comprised of the broad-spectrum antibiotic amoxicillin trihydrate and the β-lactamase inhibitor, clavulanate potassium (the potassium salt of clavulanic acid).
Amoxicillin trihydrate is a semisynthetic antibiotic with a broad spectrum of bactericidal activity against many gram-positive and gram-negative, aerobic and anaerobic microorganisms. It does not resist destruction by β-lactamases; therefore, it is not effective against β-lactamase-producing bacteria. Chemically, it is D(-)-α-amino-p-hydroxybenzyl penicillin trihydrate.
Clavulanic acid, an inhibitor of β-lactamase enzymes, is produced by the fermentation of Streptomyces clavuligerus. Clavulanic acid by itself has only weak antibacterial activity. Chemically, clavulanate potassium is potassium z-(3R,5R)-2-β-hydroxyethylidene clavam-3-carboxylate.
-
MECHANISM OF ACTION
ACTIONS:
Clavacillin is stable in the presence of gastric acid and is not significantly influenced by gastric or intestinal contents. The 2 components are rapidly absorbed resulting in amoxicillin and clavulanic acid concentrations in serum, urine, and tissues similar to those produced when each is administered alone.
Amoxicillin and clavulanic acid diffuse readily into most body tissues and fluids with the exception of brain and spinal fluid, which amoxicillin penetrates adequately when meninges are inflamed. Most of the amoxicillin is excreted unchanged in the urine. Clavulanic acid's penetration into spinal fluid is unknown at this time. Approximately 15% of the administered dose of clavulanic acid is excreted in the urine within the first 6 hours.
Clavacillin combines the distinctive properties of a broad-spectrum antibiotic and a β-lactamase inhibitor to effectively extend the antibacterial spectrum of amoxicillin to include β-lactamase-producing as well as non-β-lactamase-producing aerobic and anaerobic organisms.
-
MICROBIOLOGY
MICROBIOLOGY:
Amoxicillin is bactericidal in action and acts through the inhibition of biosynthesis of cell wall mucopeptide of susceptible organisms. The action of clavulanic acid extends the antimicrobial spectrum of amoxicillin to include organisms resistant to amoxicillin and other β-lactam antibiotics. Amoxicillin/clavulanate has been shown to have a wide range of activity which includes β-lactamase-producing strains of both gram-positive and gram-negative aerobes, facultative anaerobes, and obligate anaerobes. Many strains of the following organisms, including β-lactamase-producing strains, isolated from veterinary sources, were found to be susceptible to amoxicillin/clavulanate in vitro but the clinical significance of this activity has not been demonstrated for some of these organisms in animals.
Aerobic bacteria, including Staphylococcus aureus1, β-lactamase-producing Staphylococcus aureus1 (penicillin resistant), Staphylococcus species1, Staphylococcus epidermidis, Staphylococcus intermedius, Streptococcus faecalis, Streptococcus species1, Corynebacterium pyogenes, Corynebacterium species, Erysipelothrix rhusiopathiae, Bordetella bronchiseptica, Escherichia coli1, Proteus mirabilis, Proteus species, Enterobacter species, Klebsiella pneumoniae, Salmonella dublin, Salmonella typhimurium, Pasteurella multocida, Pasteurella haemolytica, Pasteurella species1.
Studies have demonstrated that both aerobic and anaerobic flora are isolated from gingival cultures of dogs with clinical evidence of periodontal disease. Both gram-positive and gram-negative aerobic and anaerobic subgingival isolates indicate sensitivity to amoxicillin/clavulanic acid during antimicrobial susceptibility testing.
- 1
- The susceptibility of these organisms has also been demonstrated in in vivo studies.
-
SPL UNCLASSIFIED SECTION
SUSCEPTIBILITY TEST:
The recommended quantitative disc susceptibility method (FEDERAL REGISTER 37:20527-29; Bauer AW, Kirby WMM, Sherris JC, et al: Antibiotic susceptibility testing by standardized single disc method. Am J Clin Path 45:493, 1966) utilized 30 mcg Augmentin® (AMC) discs for estimating the susceptibility of bacteria to amoxicillin and clavulanate potassium tablets.
-
VETERINARY INDICATIONS
INDICATIONS:
Clavacillin Tablets are indicated in the treatment of:
Dogs: Skin and soft tissue infections such as wounds, abscesses, cellulitis, superficial/juvenile and deep pyoderma due to susceptible strains of the following organisms: β-lactamase-producing Staphylococcus aureus, non-β-lactamase-producing Staphylococcus aureus, Staphylococcus spp., Streptococcus spp., and E. coli.
Periodontal infections due to susceptible strains of both aerobic and anaerobic bacteria. Clavacillin has been shown to be clinically effective for treating cases of canine periodontal disease.
Cats: Skin and soft tissue infections such as wounds, abscesses, and cellulitis/dermatitis due to susceptible strains of the following organisms: β-lactamase-producing Staphylococcus aureus, non-β-lactamase-producing Staphylococcus aureus, Staphylococcus spp., Streptococcus spp., E. coli, and Pasteurella spp. Urinary tract infections (cystitis) due to susceptible strains of E. coli.
Therapy may be initiated with Clavacillin prior to obtaining results from bacteriological and susceptibility studies. A culture should be obtained prior to treatment to determine susceptibility of the organisms to Clavacillin. Following determination of susceptibility results and clinical response to medication, therapy may be reevaluated.
- CONTRAINDICATIONS
- WARNINGS
-
ADVERSE REACTIONS
ADVERSE REACTIONS:
Clavacillin contains a semisynthetic penicillin (amoxicillin) and has the potential for producing allergic reactions. If an allergic reaction occurs, administer epinephrine and/or steroids.
Post-Approval Experience (July, 2017)
The following adverse events are based on post-approval adverse drug experience reporting. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported for dogs and cats are listed in decreasing order of reporting frequency for amoxicillin and clavulanate potassium tablets: Anorexia, lethargy, vomiting and diarrhea.
To report suspected adverse events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Dechra at (866) 933-2472.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or online at http://www.fda.gov/reportanimalae
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION:
Dogs: The recommended dosage is 6.25 mg/lb of body weight twice a day.
Skin and soft tissue infections such as abscesses, cellulitis, wounds, superficial/juvenile pyoderma, and periodontal infections should be treated for 5-7 days or for 48 hours after all symptoms have subsided. If no response is seen after 5 days of treatment, therapy should be discontinued and the case reevaluated. Deep pyoderma may require treatment for 21 days; the maximum duration of treatment should not exceed 30 days.
Cats: The recommended dosage is 62.5 mg twice a day.
Skin and soft tissue infections such as abscesses and cellulitis/dermatitis should be treated for 5-7 days or for 48 hours after all symptoms have subsided, not to exceed 30 days. If no response is seen after 3 days of treatment, therapy should be discontinued and the case reevaluated.
Urinary tract infections may require treatment for 10-14 days or longer. The maximum duration of treatment should not exceed 30 days.
-
HOW SUPPLIED
HOW SUPPLIED:
Clavacillin Tablets in the following strengths are supplied in strip packs. Each carton can hold 5 strips with 14 tablets (70 tablets per carton) or 15 strips with 14 tablets (210 tablets per carton).
Each 62.5-mg tablet contains amoxicillin trihydrate equivalent to 50 mg of amoxicillin activity and 12.5 mg of clavulanic acid as the potassium salt. For use in dogs and cats.
Each 125-mg tablet contains amoxicillin trihydrate equivalent to 100 mg of amoxicillin activity and 25 mg of clavulanic acid as the potassium salt. For use in dogs only.
Each 250-mg tablet contains amoxicillin trihydrate equivalent to 200 mg of amoxicillin activity and 50 mg of clavulanic acid as the potassium salt. For use in dogs only.
Each 375-mg tablet contains amoxicillin trihydrate equivalent to 300 mg of amoxicillin activity and 75 mg of clavulanic acid as the potassium salt. For use in dogs only.
Dispense according to recommendations outlined in Dosage and Administration section.
-
SPL UNCLASSIFIED SECTION
Approved by FDA under ANADA # 200-592
Augmentin is a trademark owned by GlaxoSmithKline.
Manufactured for:
Dechra Veterinary Products
7015 College Boulevard, Suite 525
Overland Park, KS 66211 USAClavacillin® is a registered trademark of Dechra Veterinary Products, LLC.
©2022. Dechra Veterinary Products, LLC.Rev. April 2022

-
PRINCIPAL DISPLAY PANEL - 62.5 mg Tablet Blister Pack Carton
Clavacillin®
(amoxicillin and clavulanate potassium tablets), USP
62.5 mgVeterinary Tablets
For use in dogs and cats
15 strips, 14 tablets each
210 tablets62.5 mg
Each film-coated tablet contains amoxicillin trihydrate
equivalent to 50 mg of amoxicillin USP activity and 12.5 mg of
clavulanic acid USP as the potassium salt.Caution: Federal law restricts this drug to use by or on the
order of a licensed veterinarian.Approved by FDA under ANADA # 200-592
Dechra

-
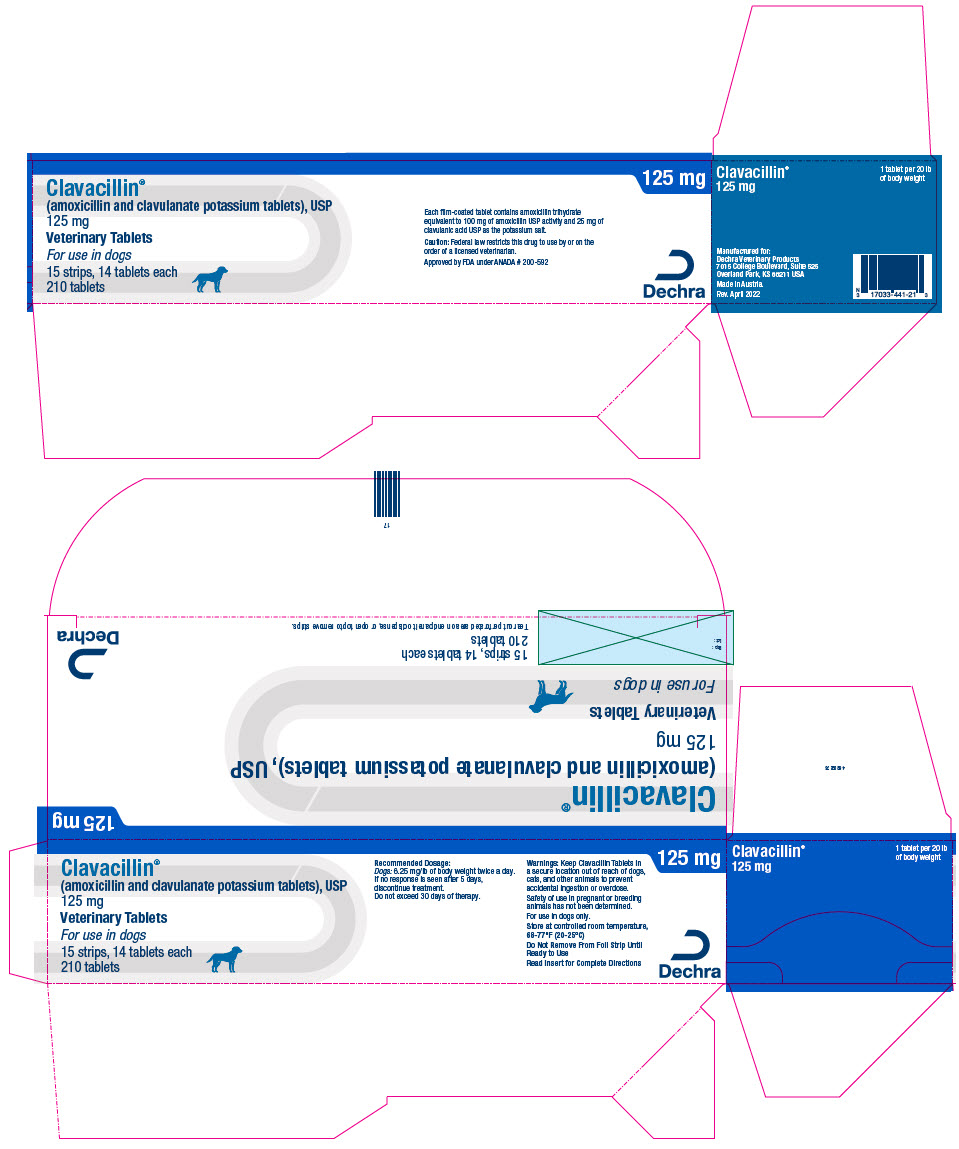
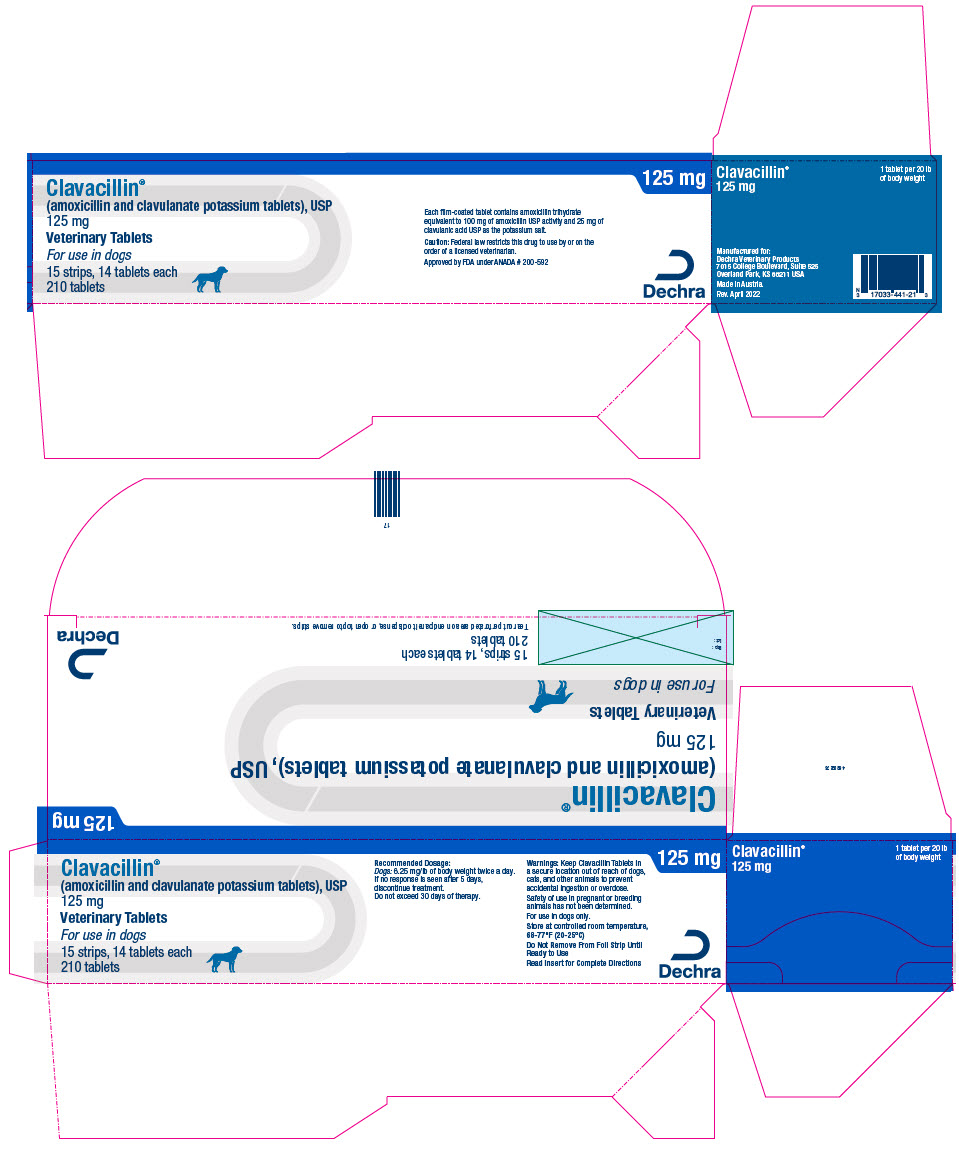
PRINCIPAL DISPLAY PANEL - 125 mg Tablet Blister Pack Carton
Clavacillin®
(amoxicillin and clavulanate potassium tablets), USP
125 mgVeterinary Tablets
For use in dogs
15 strips, 14 tablets each
210 tablets125 mg
Each film-coated tablet contains amoxicillin trihydrate
equivalent to 100 mg of amoxicillin USP activity and 25 mg of
clavulanic acid USP as the potassium salt.Caution: Federal law restricts this drug to use by or on the
order of a licensed veterinarian.Approved by FDA under ANADA # 200-592
Dechra
-
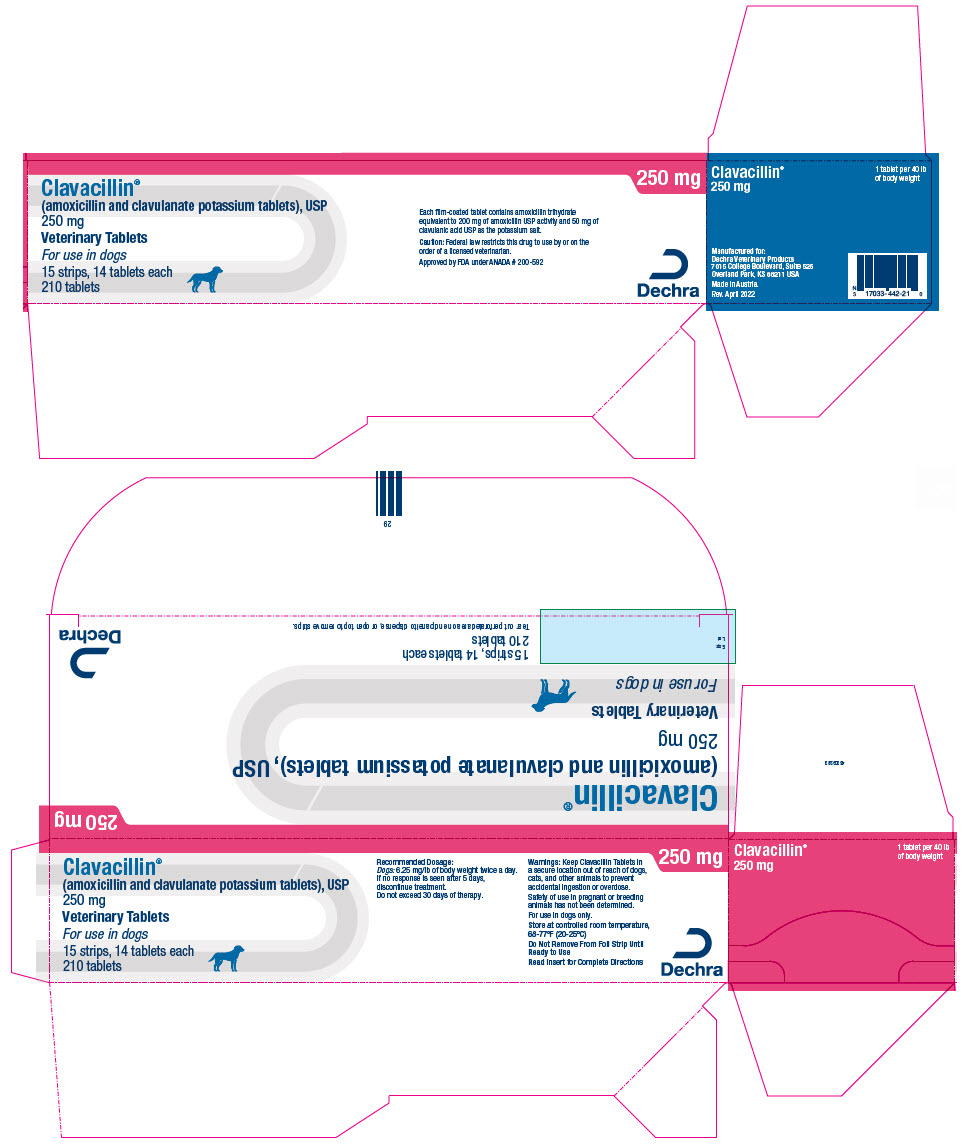
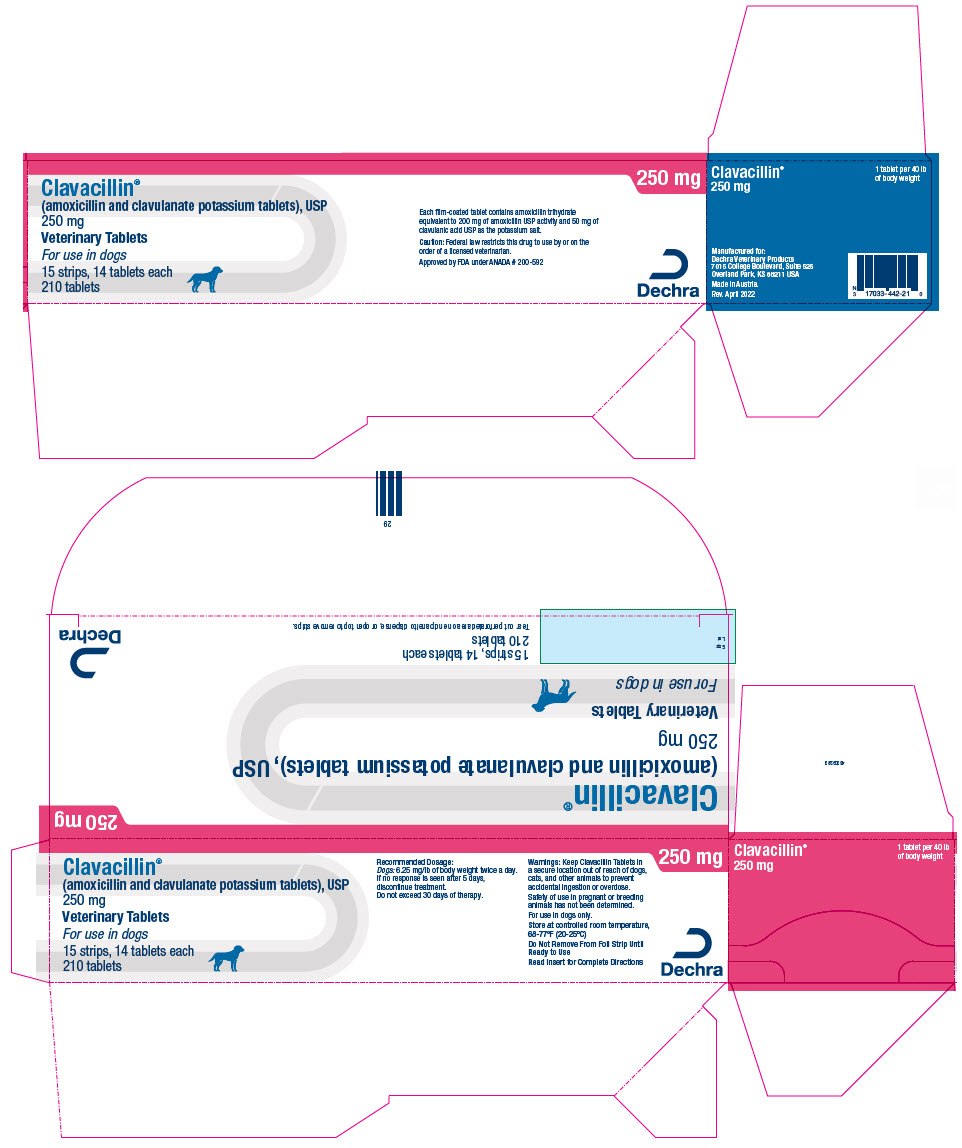
PRINCIPAL DISPLAY PANEL - 250 mg Tablet Blister Pack Carton
Clavacillin®
(amoxicillin and clavulanate potassium tablets), USP
250 mgVeterinary Tablets
For use in dogs
15 strips, 14 tablets each
210 tablets250 mg
Each film-coated tablet contains amoxicillin trihydrate
equivalent to 200 mg of amoxicillin USP activity and 50 mg of
clavulanic acid USP as the potassium salt.Caution: Federal law restricts this drug to use by or on the
order of a licensed veterinarian.Approved by FDA under ANADA # 200-592
Dechra
-
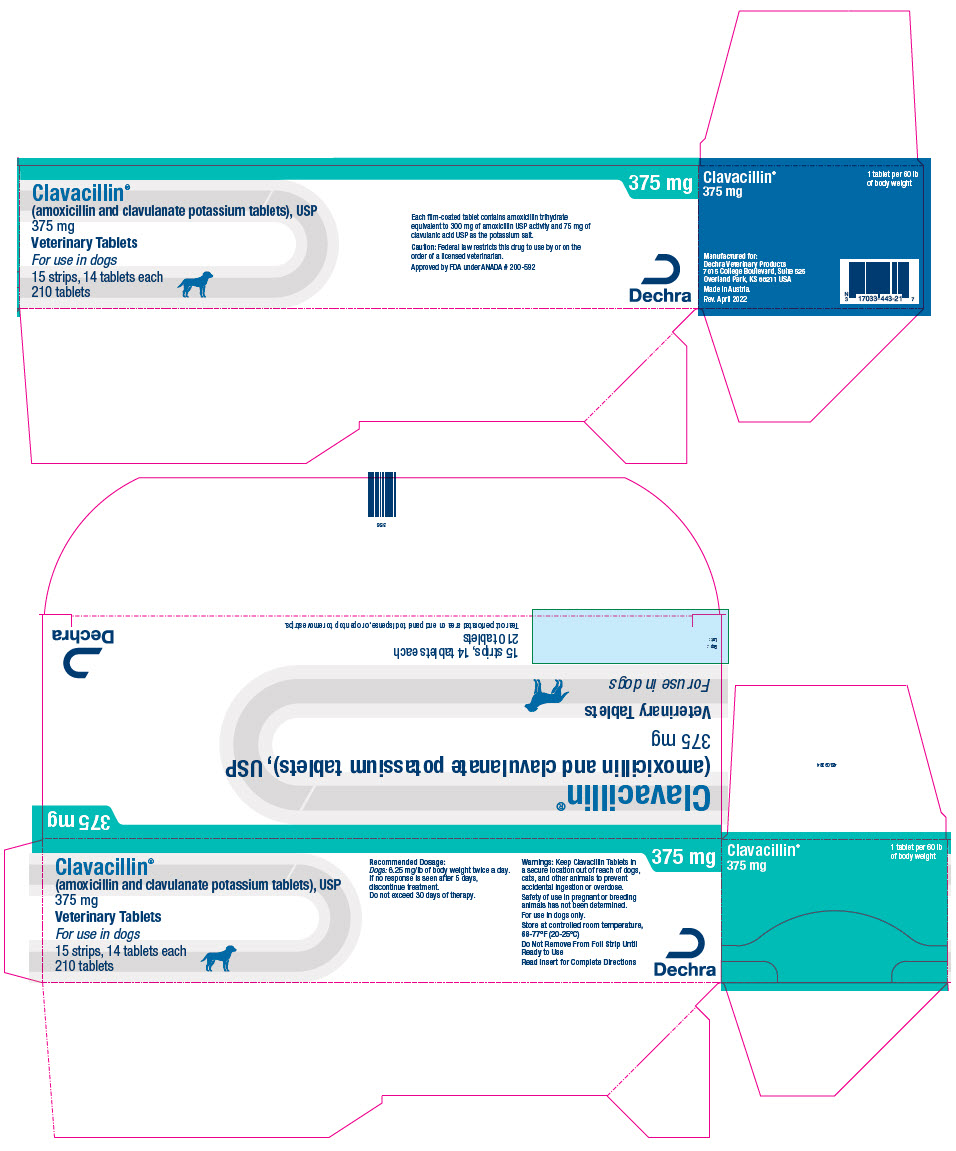
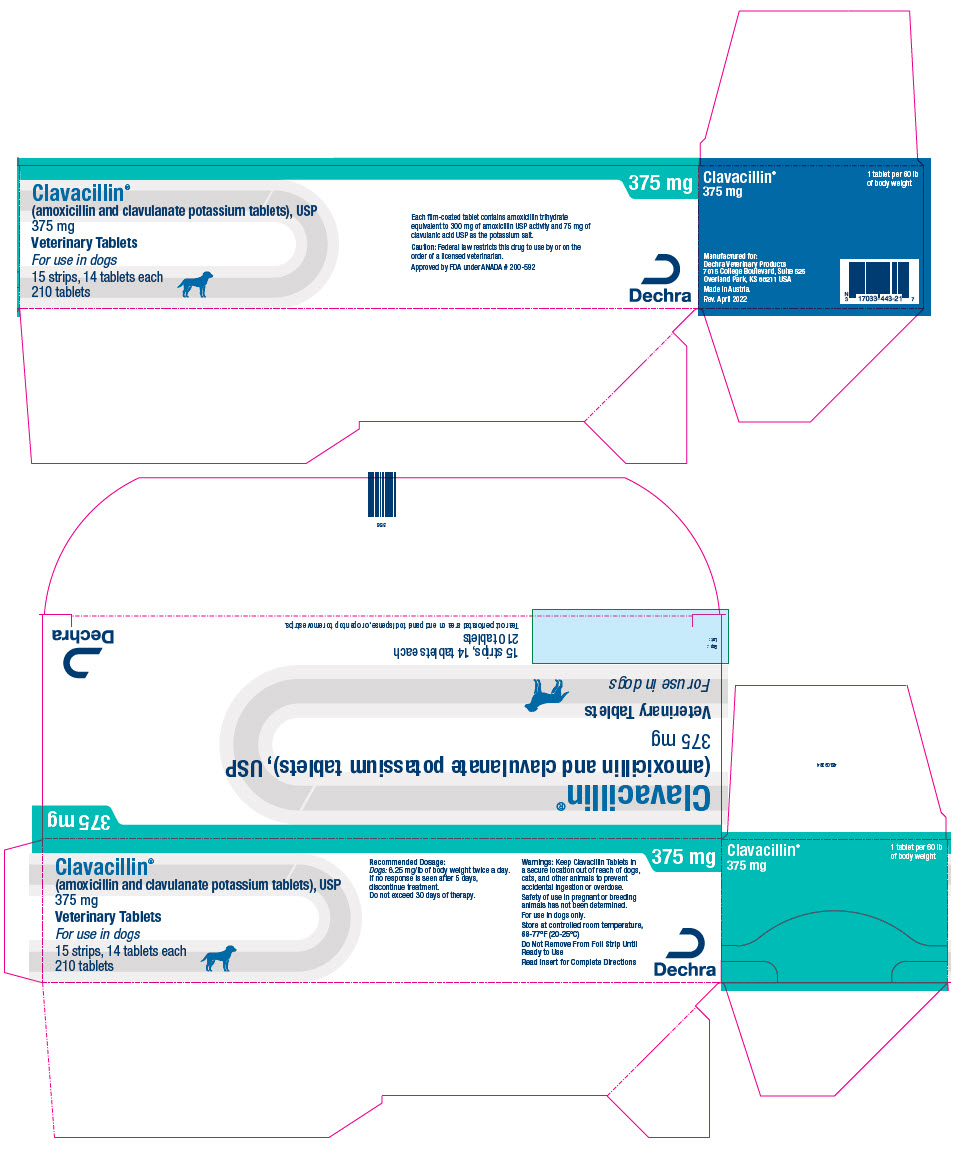
PRINCIPAL DISPLAY PANEL - 375 mg Tablet Blister Pack Carton
Clavacillin®
(amoxicillin and clavulanate potassium tablets), USP
375 mgVeterinary Tablets
For use in dogs
15 strips, 14 tablets each
210 tablets375 mg
Each film-coated tablet contains amoxicillin trihydrate
equivalent to 300 mg of amoxicillin USP activity and 75 mg of
clavulanic acid USP as the potassium salt.Caution: Federal law restricts this drug to use by or on the
order of a licensed veterinarian.Approved by FDA under ANADA # 200-592
Dechra
-
INGREDIENTS AND APPEARANCE
CLAVACILLIN
amoxicillin anhydrous and clavulanate potassium tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:17033-440 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength amoxicillin anhydrous (UNII: 9EM05410Q9) (amoxicillin anhydrous - UNII:9EM05410Q9) amoxicillin anhydrous 50 mg clavulanate potassium (UNII: Q42OMW3AT8) (clavulanic acid - UNII:23521W1S24) clavulanic acid 12.5 mg Product Characteristics Color YELLOW Score no score Shape ROUND Size 7mm Flavor Imprint Code P1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17033-440-07 5 in 1 CARTON 1 14 in 1 BLISTER PACK 2 NDC:17033-440-21 15 in 1 CARTON 2 14 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200592 01/10/2019 CLAVACILLIN
amoxicillin anhydrous and clavulanate potassium tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:17033-441 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength amoxicillin anhydrous (UNII: 9EM05410Q9) (amoxicillin anhydrous - UNII:9EM05410Q9) amoxicillin anhydrous 100 mg clavulanate potassium (UNII: Q42OMW3AT8) (clavulanic acid - UNII:23521W1S24) clavulanic acid 25 mg Product Characteristics Color YELLOW Score no score Shape ROUND Size 9mm Flavor Imprint Code P2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17033-441-07 5 in 1 CARTON 1 14 in 1 BLISTER PACK 2 NDC:17033-441-21 15 in 1 CARTON 2 14 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200592 01/10/2019 CLAVACILLIN
amoxicillin anhydrous and clavulanate potassium tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:17033-442 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength amoxicillin anhydrous (UNII: 9EM05410Q9) (amoxicillin anhydrous - UNII:9EM05410Q9) amoxicillin anhydrous 200 mg clavulanate potassium (UNII: Q42OMW3AT8) (clavulanic acid - UNII:23521W1S24) clavulanic acid 50 mg Product Characteristics Color YELLOW Score no score Shape ROUND Size 11mm Flavor Imprint Code P3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17033-442-07 5 in 1 CARTON 1 14 in 1 BLISTER PACK 2 NDC:17033-442-21 15 in 1 CARTON 2 14 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200592 01/10/2019 CLAVACILLIN
amoxicillin anhydrous and clavulanate potassium tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:17033-443 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength amoxicillin anhydrous (UNII: 9EM05410Q9) (amoxicillin anhydrous - UNII:9EM05410Q9) amoxicillin anhydrous 300 mg clavulanate potassium (UNII: Q42OMW3AT8) (clavulanic acid - UNII:23521W1S24) clavulanic acid 75 mg Product Characteristics Color YELLOW Score no score Shape ROUND Size 13mm Flavor Imprint Code P4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17033-443-07 5 in 1 CARTON 1 14 in 1 BLISTER PACK 2 NDC:17033-443-21 15 in 1 CARTON 2 14 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200592 01/10/2019 Labeler - Dechra Veterinary Products LLC (362142734)