Label: ANIMAX- nystatin-neomycin sulfate-thiostrepton-triamcinolone acetonide ointment
- NDC Code(s): 17033-122-15, 17033-122-30, 17033-122-75
- Packager: Dechra Veterinary Products
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated January 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
Animax Ointment combines nystatin, neomycin sulfate, thiostrepton and triamcinolone acetonide in a non-irritating polyethylene and mineral oil base.
- Each mL contains:
nystatin 100,000 units
neomycin sulfate equivalent to neomycin base 2.5 mg
thiostrepton 2,500 units
triamcinolone acetonide 1 mg
The preparation is intended for local therapy in a variety of cutaneous disorders of dogs and cats; it is especially useful in disorders caused, complicated, or threatened by bacterial and/or candidal (monilial) infection.
-
ACTIONS:
- By virtue of its four active ingredients, Animax Ointment provides four basic therapeutic effects: anti-inflammatory, antipruritic, antifungal and antibacterial. Triamcinolone acetonide is a potent synthetic corticosteroid providing rapid and prolonged symptomatic relief on topical administration. Inflammation, edema, and pruritus promptly subside, and lesions are permitted to heal. Nystatin is the first well-tolerated antifungal antibiotic of dependable efficacy for the treatment of cutaneous infections caused by Candida albicans (Monilia). Nystatin is fungistatic in vitro against a variety of yeast and yeast-like fungi including many fungi pathogenic to animals. No appreciable activity is exhibited against bacteria. Thiostrepton has a high order of activity against gram-positive organisms, including many which are resistant to other antibiotics; neomycin exerts antimicrobial action against a wide range of gram-positive and gram-negative bacteria. Together they provide comprehensive therapy against those organisms responsible for most superficial bacterial infections.
-
WARNINGS:
Clinical and experimental data have demonstrated that corticosteroids administered orally or by injection to animals may induce the first stage of parturition if used during the last trimester of pregnancy and may precipitate premature parturition followed by dystocia, fetal death, retained placenta, and metritis.
Additionally, corticosteroids administered to dogs, rabbits and rodents during pregnancy have resulted in cleft palate in the offspring. In dogs, other congenital anomalies have resulted: deformed forelegs, phocomelia, and anasarca.
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
-
INDICATIONS:
Animax Ointment is particularly useful in the treatment of acute and chronic otitis of varied etiologies, in interdigital cysts in cats and dogs and in anal gland infections in dogs.
The preparation is also indicated in the management of dermatologic disorders characterized by inflammation and dry or exudative dermatitis, particularly those caused, complicated, or threatened by bacterial or candidal (Candida albicans) infections. It is also of value in eczematous dermatitis, contact dermatitis, and seborrheic dermatitis; and as an adjunct in the treatment of dermatitis due to parasitic infestation.
-
PRECAUTIONS:
Animax Ointment is not intended for the treatment of deep abscesses or deep-seated infections such as inflammation of the lymphatic vessels. Parenteral antibiotic therapy is indicated in these infections.
Animax Ointment has been extremely well tolerated. Cutaneous reactions attributable to its use have been extremely rare. The occurrence of systemic reactions is rarely a problem with topical administration. There is some evidence that corticosteroids can be absorbed after topical application and cause systemic effects. Therefore, an animal receiving Animax Ointment therapy should be observed closely for signs such as polydipsia, polyuria, and increased weight gain.
Animax Ointment is not generally recommended for the treatment of deep or puncture wounds or serious burns.
Sensitivity to neomycin may occur. If redness, irritation, or swelling persists or increases, discontinue use. Do not use if pus is present since the drug may allow the infection to spread.
Avoid ingestion. Oral or parenteral use of corticosteroids (depending on dose, duration of use, and specific steroid) may result in inhibition of endogenous steroid production following drug withdrawal.
-
SIDE EFFECTS:
SAP and SGPT (ALT) enzyme elevations, polydipsia and polyuria, vomiting, and diarrhea (occasionally bloody) have been observed following parenteral or systemic use of synthetic corticosteroids in dogs.
Cushing's syndrome has been reported in association with prolonged or repeated steroid therapy in dogs. Temporary hearing loss has been reported in conjunction with treatment of otitis with products containing corticosteroids. However, regression usually occurred following withdrawal of the drug. Hearing loss, with varying degrees of recovery, has been reported with the use of Animax Ointment. If hearing dysfunction is noted during the course of treatment with Animax Ointment, discontinue its use.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the safety data sheet (SDS), contact Dechra at (866) 933-2472.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/reportanimalae.
-
CAUTION:
- Before instilling any medication into the ear, examine the external ear canal thoroughly to be certain the tympanic membrane is not ruptured in order to avoid the possibility of transmitting infection to the middle ear as well as damaging the cochlea or vestibular apparatus from prolonged contact. If hearing or vestibular dysfunction is noted during the course of treatment, discontinue the use of Animax Ointment.
-
DOSAGE AND ADMINISTRATION:
- Frequency of administration is dependent on the severity of the condition. For mild inflammations, application may range from once daily to once a week; for severe conditions Animax Ointment may be applied as often as two to three times daily, if necessary.
- Frequency of treatment may be decreased as improvement occurs.
- Wear gloves during the administration of the ointment or wash hands immediately after application.
Otitis
- Clean ear canal of impacted cerumen. Inspect canal and remove any foreign bodies such as grass awns, ticks, etc. Instill three to five drops of Animax Ointment.
- Preliminary use of a local anesthetic may be advisable.
Infected Anal Glands, Cystic Areas, etc.
- Drain gland or cyst and then fill with Animax Ointment.
Other Dermatologic Disorders
- Clean affected areas, removing any encrusted discharge or exudate. Apply Animax Ointment sparingly in a thin film.
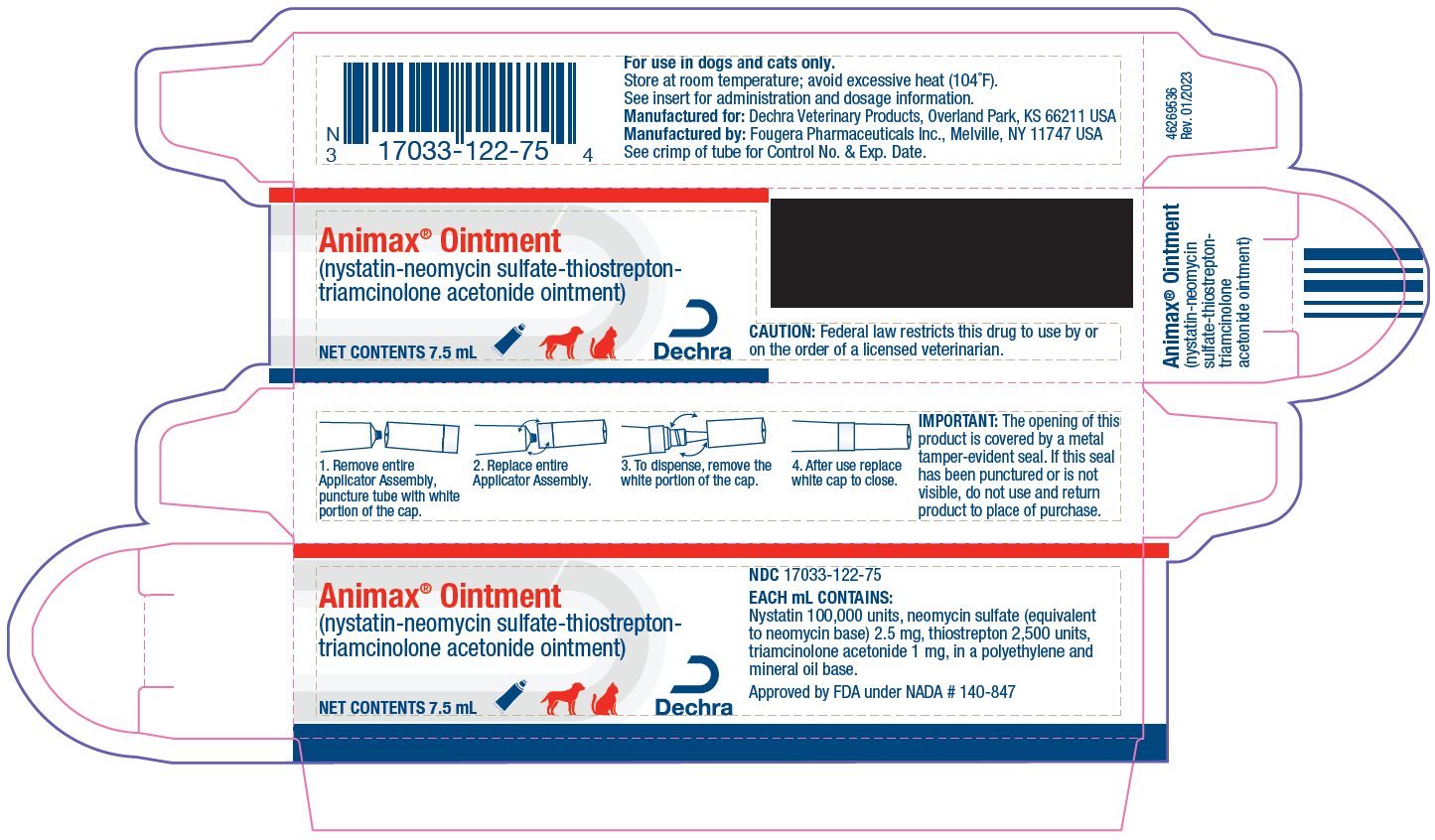
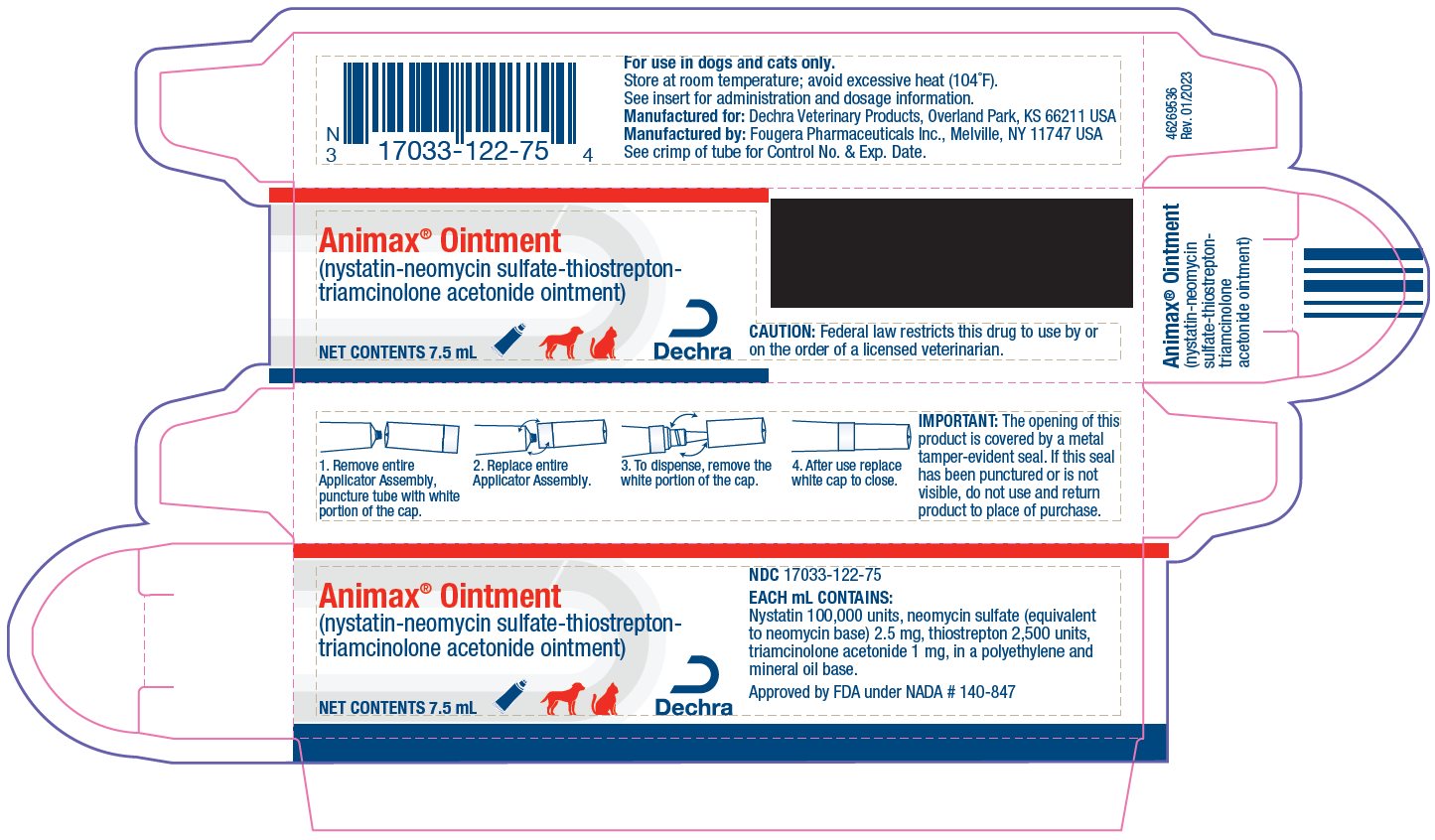
- HOW SUPPLIED:
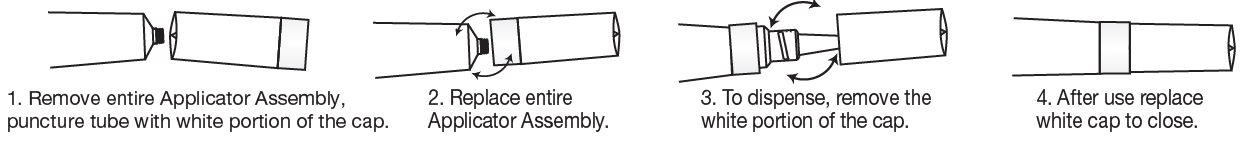
- TO OPEN:
-
IMPORTANT:
The opening of this product is covered by a metal tamper-evident seal.
If this seal has been punctured or is not visible, do not use and return product to place of purchase.
Approved by FDA under NADA # 140-847
MANUFACTURED FOR:
Dechra Veterinary ProductsOverland Park, KS 66211 USA
MANUFACTURED BY:
Fougera Pharmaceuticals Inc.Melville, NY 11747 USA
- 46269508 Rev. 01/2023
- PRINCIPAL DISPLAY PANEL - 7.5 mL Carton
-
INGREDIENTS AND APPEARANCE
ANIMAX
nystatin-neomycin sulfate-thiostrepton-triamcinolone acetonide ointmentProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:17033-122 Route of Administration TOPICAL, AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NYSTATIN (UNII: BDF1O1C72E) (NYSTATIN - UNII:BDF1O1C72E) NYSTATIN 100000 [USP'U] in 1 mL NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 2.5 mg in 1 mL THIOSTREPTON (UNII: HR4S203Y18) (THIOSTREPTON - UNII:HR4S203Y18) THIOSTREPTON 2500 [USP'U] in 1 mL TRIAMCINOLONE ACETONIDE (UNII: F446C597KA) (TRIAMCINOLONE ACETONIDE - UNII:F446C597KA) TRIAMCINOLONE ACETONIDE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17033-122-75 1 in 1 CARTON 1 7.5 mL in 1 TUBE 2 NDC:17033-122-15 1 in 1 CARTON 2 15 mL in 1 TUBE 3 NDC:17033-122-30 1 in 1 CARTON 3 30 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA140847 09/30/1988 Labeler - Dechra Veterinary Products (362142734)