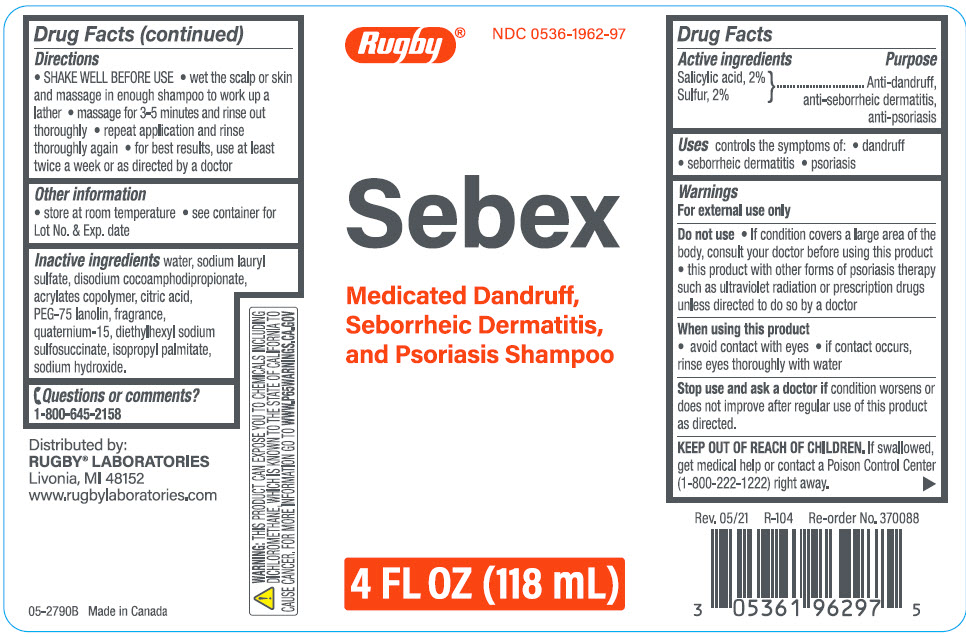
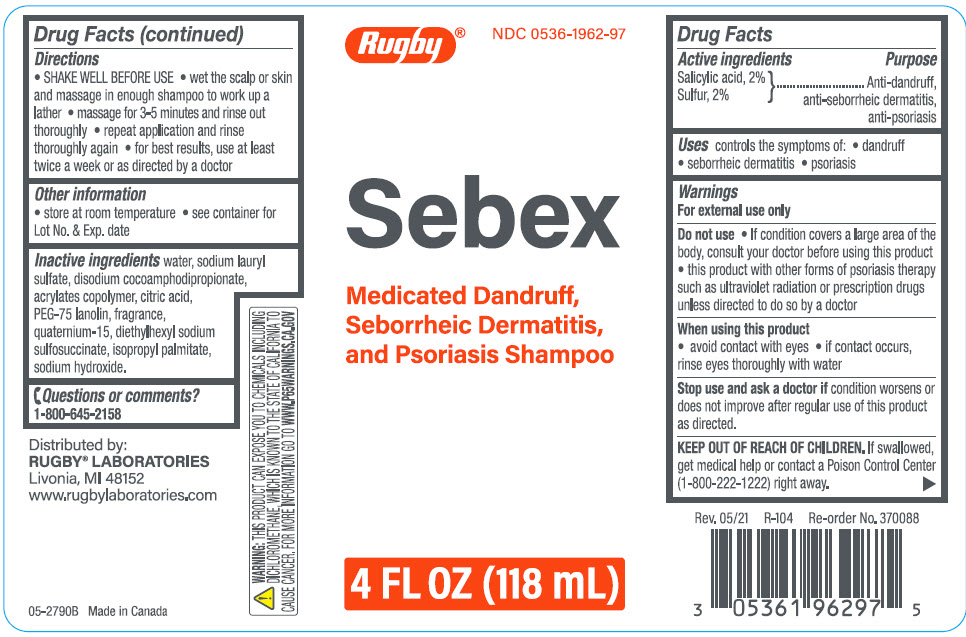
Label: SEBEX ANTIDANDRUFF, ANTI-SEBORRHEIC DERMATITIS, ANTI-PSORIASIS- salicylic acid, sulfur shampoo
- NDC Code(s): 0536-1962-97
- Packager: Rugby Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
For external use only
Do not use
- If condition covers a large area of the body, consult your doctor before using this product
- this product with other forms of psoriasis therapy such as ultraviolet radiation or prescription drugs unless directed to do so by a doctor
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 118 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
SEBEX ANTIDANDRUFF, ANTI-SEBORRHEIC DERMATITIS, ANTI-PSORIASIS
salicylic acid, sulfur shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0536-1962 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength salicylic acid (UNII: O414PZ4LPZ) (salicylic acid - UNII:O414PZ4LPZ) salicylic acid 20 mg in 1000 mL sulfur (UNII: 70FD1KFU70) (sulfur - UNII:70FD1KFU70) sulfur 20 mg in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Sodium Lauryl Sulfate (UNII: 368GB5141J) Disodium Cocoamphodipropionate (UNII: 6K8PRP397M) Citric Acid Monohydrate (UNII: 2968PHW8QP) PEG-75 Lanolin (UNII: 09179OX7TB) Quaternium-15 (UNII: E40U03LEM0) Docusate Sodium (UNII: F05Q2T2JA0) Isopropyl Palmitate (UNII: 8CRQ2TH63M) Sodium Hydroxide (UNII: 55X04QC32I) Product Characteristics Color YELLOW Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0536-1962-97 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/29/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M032 10/29/2012 Labeler - Rugby Laboratories (079246066) Registrant - Garcoa, Inc. (036464697) Establishment Name Address ID/FEI Business Operations Sigan Industries, Inc. 255106239 MANUFACTURE(0536-1962)