Label: CETACAINE TOPICAL ANESTHETIC- benzocaine, butamben, and tetracaine hydrochloride gel
- NDC Code(s): 10223-0217-3, 10223-0221-1
- Packager: Cetylite Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 17, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
-
Action
The onset of Cetacaine Topical Anesthetic Gel produced anesthesia is rapid (approximately 30 seconds) and the duration of anesthesia is typically 30-60 minutes, when used as directed. This effect is due to the rapid onset, but short duration of action of Benzocaine coupled with the slow onset, but extended duration of Tetracaine HCl and bridged by the intermediate action of Butamben.
It is believed that all of these agents act by reversibly blocking nerve conduction. Speed and duration of action is determined by the ability of the agent to be absorbed by the mucous membrane and nerve sheath and then to diffuse out, and ultimately be metabolized (primarily by plasma cholinesterases) to inert metabolites which are excreted in the urine.
-
Indications
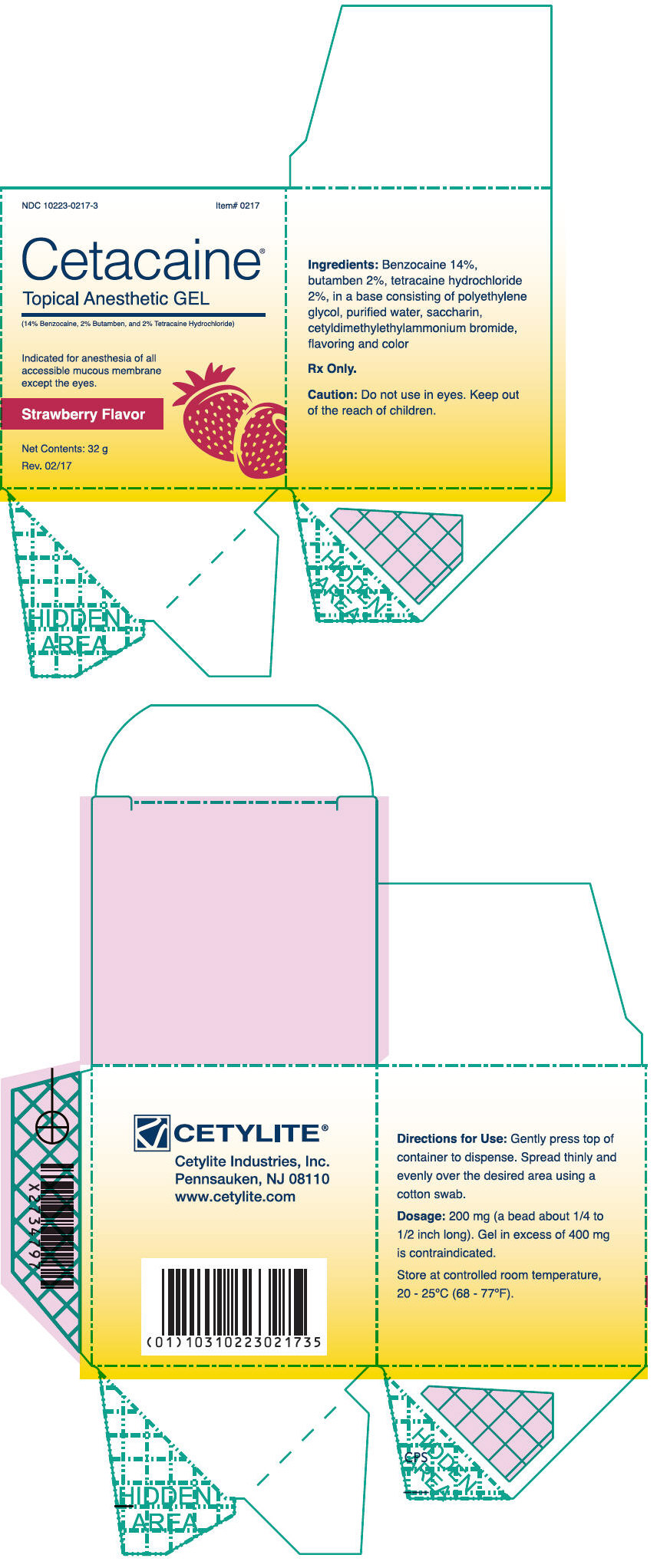
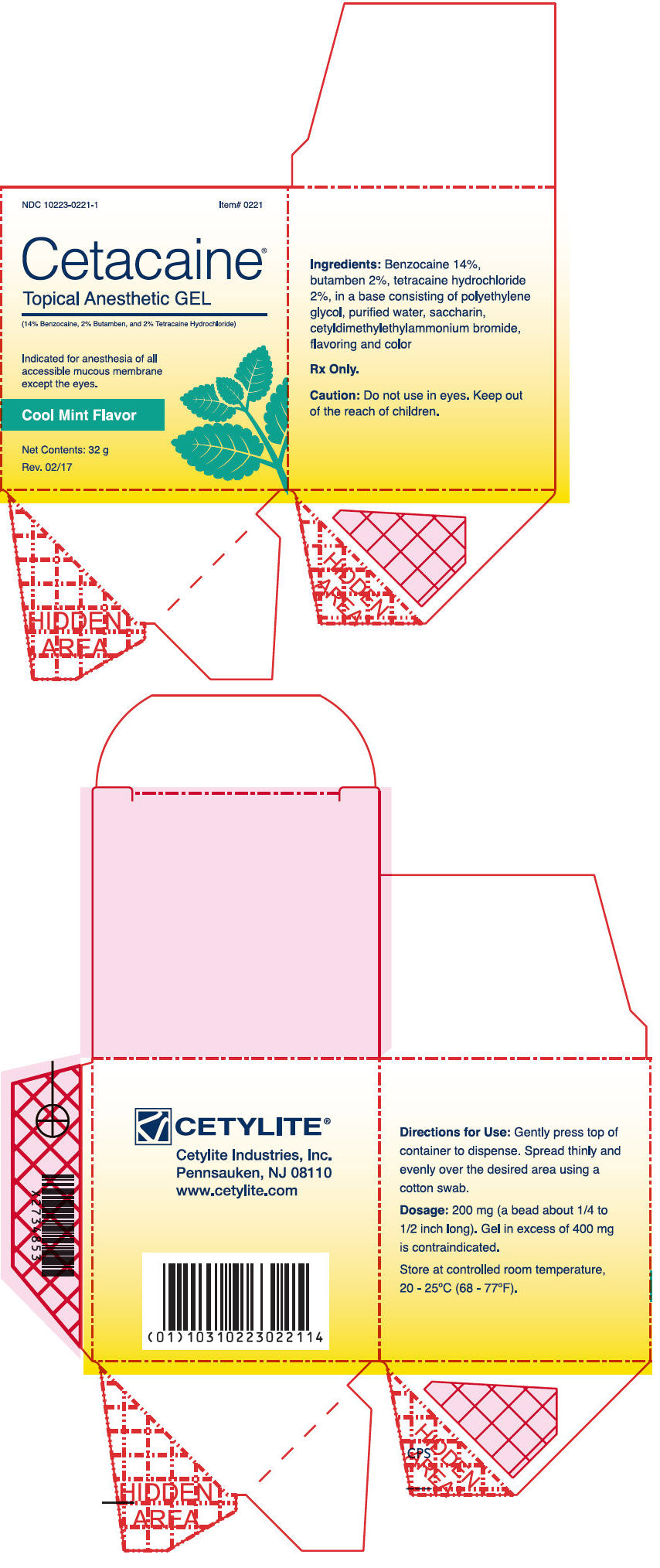
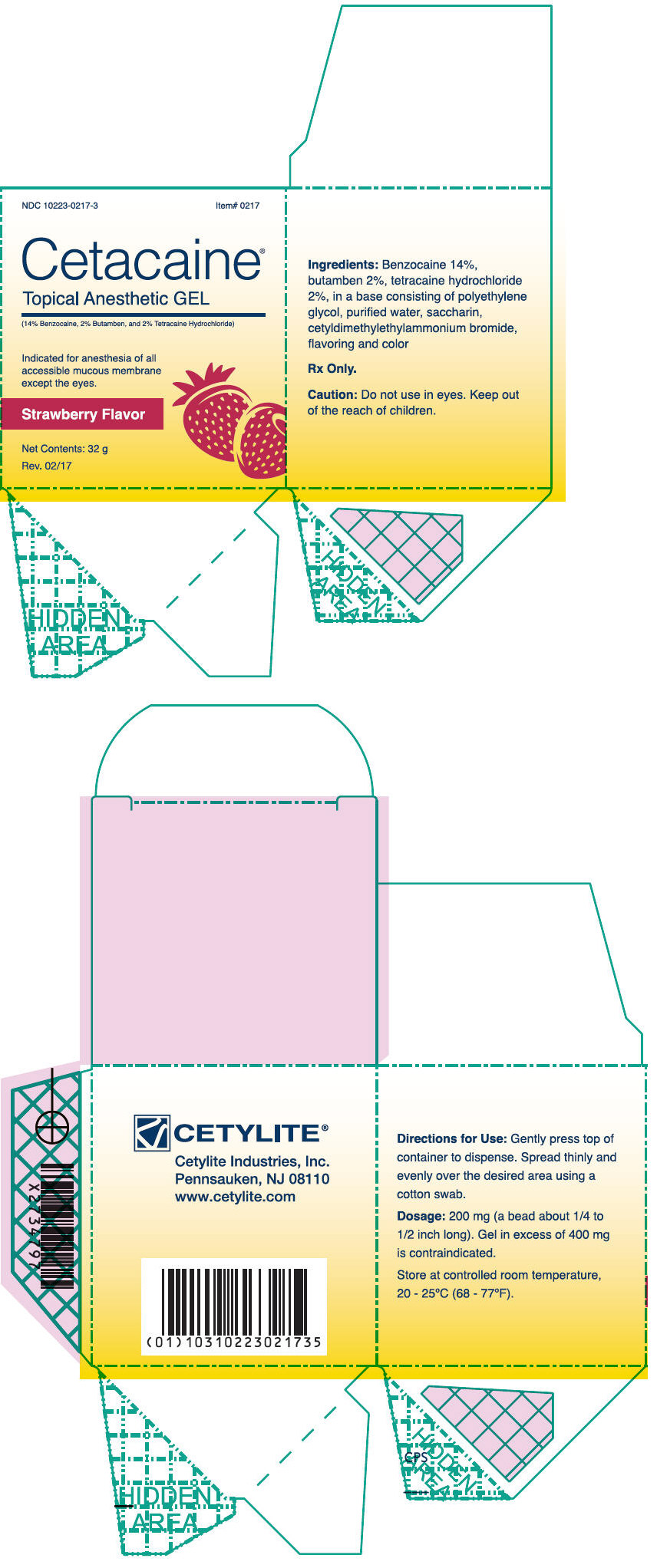
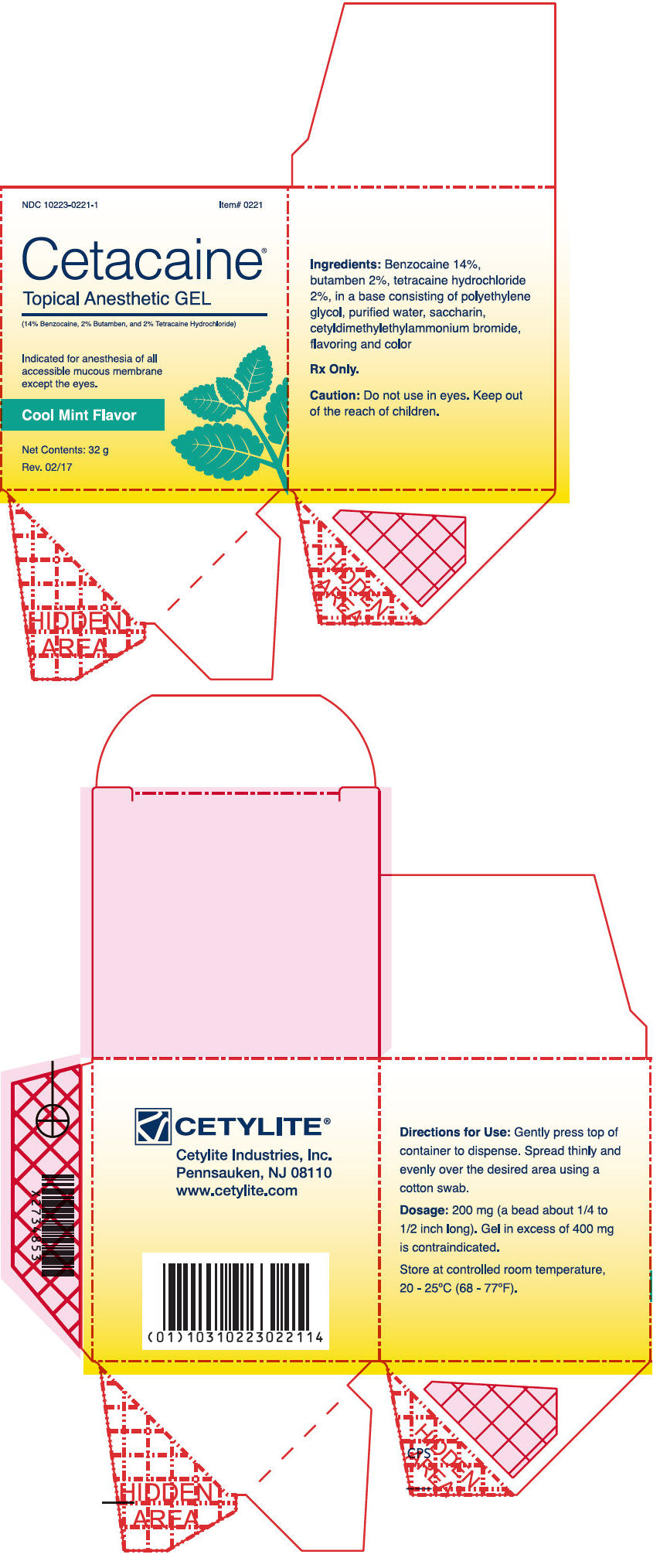
Cetacaine Topical Anesthetic Gel is a topical anesthetic indicated for the production of anesthesia of all accessible mucous membrane except the eyes. Cetacaine Topical Anesthetic Gel is indicated for use to control pain and for use for surgical or endoscopic procedures, or other procedures in the ear, nose, mouth, pharynx, larynx, trachea, bronchi, and esophagus. It may also be used for vaginal or rectal procedures where feasible.
-
Dosage and Administration
Only a limited quantity of Cetacaine Topical Anesthetic Gel is required for anesthesia.
Dispense 200 mg of gel (a bead approximately 1/4 to 1/2 inches long) by gently depressing the pump. Dispensing a bead of gel in excess of 400 mg is contraindicated. Spread thinly and evenly over the desired area using a cotton swab.
In the unlikely event that a Cetacaine Gel pump jar won't dispense, attempt the following:
- Using a gloved hand, depress the pump fully using the thumb and middle finger.
- While depressed, cover the center orifice with the index finger.
- With the orifice still covered, slowly allow the pump to return to its original starting position.
- Repeat until Cetacaine Gel is dispensed (usually about 3-4 repeated attempts).
An appropriate pediatric dosage has not been established for Cetacaine Topical Anesthetic Gel.
Dosages should be reduced in the debilitated elderly, acutely ill, and very young patients.
Do not use Cetacaine Gel to treat infants or children younger than 2 years.
Tissue need not be dried prior to application of Cetacaine Topical Anesthetic Gel.
Cetacaine Topical Anesthetic Gel should be applied directly to the site where pain control is required. Anesthesia is produced in approximately 30 seconds with an approximate duration of thirty to sixty minutes. Each 200 mg dose of Cetacaine Topical Anesthetic Gel contains 28 mg of benzocaine, 4 mg of butamben and 4 mg of tetracaine HCl.
-
WARNINGS AND PRECAUTIONS
Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs and symptoms of methemoglobinemia may occur immediately or may be delayed some hours after exposure and are characterized by a cyanotic skin discoloration and abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue Cetacaine and any other oxidizing agents. Depending on the severity of the symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration.
More severe symptoms may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
-
DRUG INTERACTIONS
Patients that are administered local anesthetics may be at increased risk of developing methemoglobinemia when concurrently exposed to the following oxidizing agents:
Class Examples Nitrates/Nitrites nitroglycerin, nitroprusside, nitric oxide, nitrous oxide Local anesthetics benzocaine, lidocaine, bupivacaine, mepivacaine, tetracaine, prilocaine, procaine, articaine, ropivacaine Antineoplastic agents cyclophosphamide, flutamide, rasburicase, ifosfamide, hydroxyurea Antibiotics dapsone, sulfonamides, nitrofurantoin, para-aminosalicylic acid Antimalarials chloroquine, primaquine Anticonvulsants phenytoin, sodium valproate, phenobarbital Other drugs acetaminophen, metoclopramide, sulfa drugs (i.e., sulfasalazine), quinine -
PATIENT COUNSELING INFORMATION
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to stop use and seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
Hypersensitivity Reactions
Unpredictable adverse reactions (i.e. hypersensitivity, including anaphylaxis) are extremely rare. Localized allergic reactions may occur after prolonged or repeated use of any aminobenzoate anesthetic. The most common adverse reaction caused by local anesthetics is contact dermatitis characterized by erythema and pruritus that may progress to vesiculation and oozing. This occurs most commonly in patients following prolonged self-medication, which is contraindicated. If rash, urticaria, edema, or other manifestations of allergy develop during use, the drug should be discontinued. To minimize the possibility of a serious allergic reaction, Cetacaine Topical Anesthetic Gel should not be applied for prolonged periods except under continual supervision. Dehydration of the epithelium or an escharotic effect may also result from prolonged contact.
Use in Pregnancy
Safe use of Cetacaine Topical Anesthetic Gel has not been established with respect to possible adverse effects upon fetal development. Therefore, Cetacaine Topical Anesthetic Gel should not be used during early pregnancy, unless in the judgement of a physician, the potential benefits outweigh the unknown hazards. Routine precaution for the use of any topical anesthetic should be observed when Cetacaine Topical Anesthetic Gel is used.
-
Contraindications
Do not use Cetacaine Gel to treat infants or children younger than 2 years. Cetacaine is not suitable and should never be used for injection. Do not use on the eyes. To avoid excessive systemic absorption, Cetacaine Topical Anesthetic Gel should not be applied to large areas of denuded or inflamed tissue. Cetacaine Topical Anesthetic Gel should not be administered to patients who are hypersensitive to any of its ingredients or to patients known to have cholinesterase deficiencies. Tolerance may vary with status of the patient.
Cetacaine Topical Anesthetic Gel should not be used under dentures or cotton rolls, as retention of the active gel ingredients under a denture or cotton roll could possibly cause an escharotic effect. Routine precaution for the use of any topical anesthetic should be observed when using Cetacaine Topical Anesthetic Gel.
- How Supplied
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 32 g Jar Box - Strawberry
- PRINCIPAL DISPLAY PANEL - 32 g Jar Box - Mint
-
INGREDIENTS AND APPEARANCE
CETACAINE TOPICAL ANESTHETIC
benzocaine, butamben, and tetracaine hydrochloride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:10223-0217 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzocaine (UNII: U3RSY48JW5) (Benzocaine - UNII:U3RSY48JW5) Benzocaine 0.028 g in 0.2 g Butamben (UNII: EFW857872Q) (Butamben - UNII:EFW857872Q) Butamben 0.004 g in 0.2 g Tetracaine Hydrochloride (UNII: 5NF5D4OPCI) (Tetracaine - UNII:0619F35CGV) Tetracaine Hydrochloride 0.004 g in 0.2 g Inactive Ingredients Ingredient Name Strength Polyethylene Glycol, Unspecified (UNII: 3WJQ0SDW1A) Saccharin (UNII: FST467XS7D) Benzalkonium chloride (UNII: F5UM2KM3W7) Mecetronium bromide (UNII: 7ZNQ7FIO9K) Water (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor STRAWBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10223-0217-3 1 in 1 BOX 01/01/1960 1 32 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 01/01/1960 CETACAINE TOPICAL ANESTHETIC
benzocaine, butamben, and tetracaine hydrochloride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:10223-0221 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzocaine (UNII: U3RSY48JW5) (Benzocaine - UNII:U3RSY48JW5) Benzocaine 0.028 g in 0.2 g Butamben (UNII: EFW857872Q) (Butamben - UNII:EFW857872Q) Butamben 0.004 g in 0.2 g Tetracaine Hydrochloride (UNII: 5NF5D4OPCI) (Tetracaine - UNII:0619F35CGV) Tetracaine Hydrochloride 0.004 g in 0.2 g Inactive Ingredients Ingredient Name Strength Polyethylene Glycol, Unspecified (UNII: 3WJQ0SDW1A) Saccharin (UNII: FST467XS7D) Benzalkonium chloride (UNII: F5UM2KM3W7) Mecetronium bromide (UNII: 7ZNQ7FIO9K) Water (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10223-0221-1 1 in 1 BOX 01/01/1960 1 32 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 01/01/1960 Labeler - Cetylite Industries, Inc. (001283704) Establishment Name Address ID/FEI Business Operations Cetylite Industries, Inc. 001283704 MANUFACTURE(10223-0217, 10223-0221) , ANALYSIS(10223-0217, 10223-0221) , LABEL(10223-0217, 10223-0221) , PACK(10223-0217, 10223-0221)