Label: CROTAN- crotamiton lotion
- NDC Code(s): 0682-0051-10, 0682-0051-20, 0682-0051-30
- Packager: Marnel Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS AND PRECAUTIONS
-
INDICATIONS & USAGE
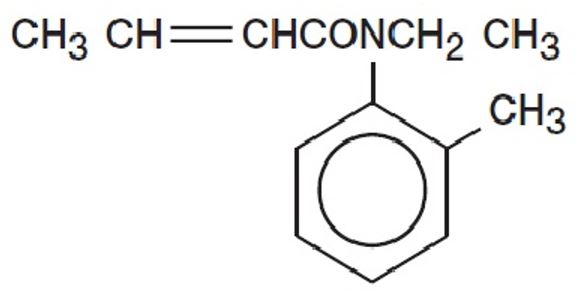
CROTAN ™ (crotamiton USP 10%) is a scabicidal and antipruritic agent as a lotion for topical use only. Crotamiton is a colorless to slightly yellowish oil, having a faint amine-like odor. It is miscible with alcohol and with methanol. Crotamiton is a mixture of the cis and trans isomers. Its molecular weight is 203. 28. Crotamiton is N-ethyl-N(o-methyl-phenyl) 2-butenamide and its structural formula is:
CROTAN lotion contains crotamiton USP 10% (100mg/ml) in a creamy lotion base containing purified water, light mineral oil, propylene glycol, cetearyl alcohol (and) cetearth-20, cetyl alcohol, lanolin, benzyl alcohol, carbomer 971P, sodium hydroxide with citric acid (for pH adjustment).
- CLINICAL PHARMACOLOGY
-
GERIATRIC USE
Geriatric Use: Clinical studies with CROTAN (crotamiton USP) lotion did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients, but great sensitivity of some older individuals cannot be ruled out.
- ADVERSE REACTIONS
-
OVERDOSAGE
OVERDOSAGE: There is no specific informaton on the effect of overtreatment with repeated topical applications in humans. A death was reported but cause was not confirmed. Accidental oral ingestion may be accompanied by burning sensation in the mouth, irritation of the buccal, esophageal and gastric mucosa, nausea, vomiting, abdominal pain.
If accidental ingestion occurs, call your Poison Control Center.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION:
SHAKE WELL BEFORE USE.
In Scabies: Thoroughly massage into the skin of the whole body, from the chin down, paying particular attention to all folds and creases. A second application is advisable 24 hours later. Clothing and bed linen should be changed the next morning. A cleansing bath should be taken 48 hours after the last application.
In Pruritis: Massage gently into affected areas until medication is completely absorbed. Repeat as needed.
DIRECTIONS FOR PATIENTS WITH SCABIES:
1. Take a routine bath or shower. Thoroughly massage CROTAN ™ lotion into the skin from the chin to the toes including folds and creases.
2. Put CROTAN lotion under fingernails after trimming the fingernails short, because scabies are likely to remain there. A toothbrush can be used to apply the CROTAN lotion under the fingernails. Immediately after use, the toothbrush should be wrapped in paper and thrown away. Use of the brush in the mouth could lead to lead poisoning.
3. A second application is advisable 24 hours leater.
4. Clothing and bed linen should be changed the next day. Contaminated clothing and bed linen may be dry-cleaned or washed in the hot cycle of the washing machine.
5. A cleansing bath should be taken 48 hours after the last application.
- INDICATIONS & USAGE
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
-
PREGNANCY
Pregnancy (Category C): Animal reproduction studies have not been conducted with CROTAN (crotamiton USP) lotion. It is also not known whether CROTAN can cause fetal harm when applied to a pregnant woman or can affect reproduction capacity. CROTAN should be given to a pregnant woman only if clearly needed.
- PEDIATRIC USE
- HOW SUPPLIED
- KEEP OUT OF REACH OF CHILDREN
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CROTAN
crotamiton lotionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0682-0051 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CROTAMITON (UNII: D6S4O4XD0H) (CROTAMITON - UNII:D6S4O4XD0H) CROTAMITON 100 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MINERAL OIL (UNII: T5L8T28FGP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CETYL ALCOHOL (UNII: 936JST6JCN) LANOLIN (UNII: 7EV65EAW6H) BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOMER HOMOPOLYMER TYPE A (UNII: F68VH75CJC) SODIUM HYDROXIDE (UNII: 55X04QC32I) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0682-0051-10 237 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/15/2022 2 NDC:0682-0051-20 60 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/15/2022 3 NDC:0682-0051-30 454 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/15/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA087204 01/15/2022 Labeler - Marnel Pharmaceuticals, Inc. (080161449) Registrant - Marnel Pharmaceuticals, Inc. (080161449) Establishment Name Address ID/FEI Business Operations Groupe Parima 252437850 manufacture(0682-0051) , pack(0682-0051)


