Label: SUBLOCADE- buprenorphine solution
-
NDC Code(s):
12496-0100-1,
12496-0100-2,
12496-0100-5,
12496-0300-1, view more12496-0300-2, 12496-0300-5
- Packager: Indivior Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIII
- Marketing Status: New Drug Application
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SUBLOCADE® safely and effectively. See full prescribing information for SUBLOCADE.
SUBLOCADE (buprenorphine extended-release) injection, for subcutaneous use, CIII
Initial U.S. Approval: 2002WARNING: RISK OF SERIOUS HARM OR DEATH WITH INTRAVENOUS ADMINISTRATION; SUBLOCADE RISK EVALUATION AND MITIGATION STRATEGY
See full prescribing information for complete boxed warning.
- Serious harm or death could result if administered intravenously. (5.1)
- SUBLOCADE is only available through a restricted program called the SUBLOCADE REMS Program. Healthcare settings and pharmacies that order and dispense SUBLOCADE must be certified in this program and comply with the REMS requirements. (5.2)
RECENT MAJOR CHANGES
Dosage and Administration (2.5) 09/2023 INDICATIONS AND USAGE
SUBLOCADE contains buprenorphine, a partial opioid agonist, and is indicated for the treatment of moderate to severe opioid use disorder in patients who have initiated treatment with a buprenorphine-containing product, followed by dose adjustment for a minimum of 7 days. (1)
SUBLOCADE should be used as part of a complete treatment program that includes counseling and psychosocial support. (1)
DOSAGE AND ADMINISTRATION
SUBLOCADE should only be prepared and administered by a healthcare provider. (2.1)
SUBLOCADE is administered monthly only by subcutaneous injection in the abdominal region. (2.1)
Strongly consider prescribing naloxone at the time SUBLOCADE is initiated or renewed because patients being treated for opioid use disorder have the potential for relapse, putting them at risk for opioid overdose. (2.2)
The recommended dose of SUBLOCADE is two monthly initial doses of 300 mg followed by 100 mg monthly maintenance doses. (2.3)
Increasing the maintenance dose to 300 mg monthly may be considered for patients in which the benefits outweigh the risks. (2.3)
Examine the injection site for signs of infection or evidence of tampering or attempts to remove the depot. (2.4)
See Full Prescribing Information for administration instructions. (2.5)
DOSAGE FORMS AND STRENGTHS
Injection: 100 mg/0.5 mL and 300 mg/1.5 mL provided in a prefilled syringe with a 19 Gauge 5/8-inch needle. (3)
CONTRAINDICATIONS
Hypersensitivity to buprenorphine or any other ingredients in SUBLOCADE. (4)
WARNINGS AND PRECAUTIONS
Addiction, Abuse, and Misuse: Buprenorphine can be abused in a manner similar to other opioids. Monitor patients for conditions indicative of diversion or progression of opioid dependence and addictive behaviors. (5.3)
Respiratory Depression: Life-threatening respiratory depression and death have occurred in association with buprenorphine. Warn patients of the potential danger of self-administration of benzodiazepines or other CNS depressants while under treatment with SUBLOCADE. (5.4, 5.5)
Risk of Serious Injection Site Reactions: Likelihood may be increased with inadvertent intramuscular or intradermal administration. Evaluate and treat as appropriate. (5.6).
Neonatal Opioid Withdrawal Syndrome: Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy. (5.7)
Adrenal Insufficiency: If diagnosed, treat with physiologic replacement of corticosteroids, and wean patient off of the opioid. (5.8)
Risk of Opioid Withdrawal With Abrupt Discontinuation: If treatment with SUBLOCADE is discontinued, monitor patients for several months for withdrawal and treat appropriately. (5.9)
Risk of Hepatitis, Hepatic Events: Monitor liver function tests prior to and during treatment. (5.10)
Risk of Withdrawal in Patients Dependent on Full Agonist Opioids:
Verify that patient is clinically stable on transmucosal buprenorphine before injecting SUBLOCADE. (5.12)
Treatment of Emergent Acute Pain: Treat pain with a non-opioid analgesic whenever possible. If opioid therapy is required, monitor patients closely because higher doses may be required for analgesic effect. (5.13)
ADVERSE REACTIONS
Adverse reactions commonly associated with SUBLOCADE (in ≥ 5% of subjects) were constipation, headache, nausea, injection site pruritus, vomiting, increased hepatic enzymes, fatigue, and injection site pain. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Indivior Inc. at 1-877-782-6966 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
CYP3A4 Inhibitors and Inducers: Monitor patients starting or ending CYP3A4 inhibitors or inducers for potential over- or under-dosing. (7)
Serotonergic Drugs: If concomitant use is warranted, monitor for serotonin syndrome, particularly during treatment initiation, and during dose adjustment of the serotonergic drug. (7)
USE IN SPECIFIC POPULATIONS
Lactation: Buprenorphine passes into the mother's milk. (8.2)
Geriatric Patients: Monitor for sedation or respiratory depression. (8.5)
Moderate to Severe Hepatic Impairment: Not recommended. (5.15, 8.6)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF SERIOUS HARM OR DEATH WITH INTRAVENOUS ADMINISTRATION; SUBLOCADE RISK EVALUATION AND MITIGATION STRATEGY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing and Administration Information
2.2 Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
2.3 Recommended Dosing
2.4 Clinical Supervision
2.5 Instructions for Use
2.6 Limits on Distribution
2.7 Removal of the Depot
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Serious Harm or Death With Intravenous Administration
5.2 SUBLOCADE Risk Evaluation and Mitigation Strategy (REMS) Program
5.3 Addiction, Abuse, and Misuse
5.4 Risk of Life-Threatening Respiratory and Central Nervous System (CNS) Depression
5.5 Managing Risks From Concomitant Use of Benzodiazepines or Other CNS Depressants With Buprenorphine
5.6 Risk of Serious Injection Site Reactions
5.7 Neonatal Opioid Withdrawal Syndrome
5.8 Adrenal Insufficiency
5.9 Risk of Opioid Withdrawal With Abrupt Discontinuation of SUBLOCADE Treatment
5.10 Risk of Hepatitis, Hepatic Events
5.11 Hypersensitivity Reactions
5.12 Precipitation of Opioid Withdrawal in Patients Dependent on Full Agonist Opioids
5.13 Risks Associated With Treatment of Emergent Acute Pain
5.14 Use in Opioid Naïve Patients
5.15 Use in Patients With Impaired Hepatic Function
5.16 QTc Prolongation
5.17 Impairment of Ability to Drive or Operate Machinery
5.18 Orthostatic Hypotension
5.19 Elevation of Cerebrospinal Fluid Pressure
5.20 Elevation of Intracholedochal Pressure
5.21 Effects in Acute Abdominal Conditions
5.22 Unintentional Pediatric Exposure
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Study 13-0002, NCT02044094
14.2 Study 13-0001, NCT02357901
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF SERIOUS HARM OR DEATH WITH INTRAVENOUS ADMINISTRATION; SUBLOCADE RISK EVALUATION AND MITIGATION STRATEGY
- Serious harm or death could result if administered intravenously. SUBLOCADE forms a solid mass upon contact with body fluids and may cause occlusion, local tissue damage, and thrombo-embolic events, including life threatening pulmonary emboli, if administered intravenously. (5.1)
- Because of the risk of serious harm or death that could result from intravenous self-administration, SUBLOCADE is only available through a restricted program called the SUBLOCADE REMS Program. Healthcare settings and pharmacies that order and dispense SUBLOCADE must be certified in this program and comply with the REMS requirements. (5.2)
-
1 INDICATIONS AND USAGE
SUBLOCADE is indicated for the treatment of moderate to severe opioid use disorder in patients who have initiated treatment with a buprenorphine-containing product, followed by dose adjustment for a minimum of 7 days.
SUBLOCADE should be used as part of a complete treatment plan that includes counseling and psychosocial support.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing and Administration Information
FOR ABDOMINAL SUBCUTANEOUS INJECTION ONLY. DO NOT ADMINISTER SUBLOCADE INTRAVENOUSLY, INTRAMUSCULARLY OR INTRADERMALLY [see Dosage and Administration (2.5), Warnings and Precautions (5.1, 5.6)].
- Only healthcare providers should prepare and administer SUBLOCADE.
- Administer SUBLOCADE monthly with a minimum of 26 days between doses.
- Initiating treatment with SUBLOCADE as the first buprenorphine product has not been studied. Initiate SUBLOCADE treatment only following induction and dose-adjustment with a transmucosal buprenorphine-containing product [see Dosage and Administration (2.3)].
- Administer each injection only using the syringe and safety needle included with the product [see Dosage and Administration (2.5)].
2.2 Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver. Because patients being treated for opioid use disorder have the potential for relapse, putting them at risk for opioid overdose, strongly consider prescribing naloxone for the emergency treatment of opioid overdose, both when initiating and renewing treatment with SUBLOCADE. Also consider prescribing naloxone if the patient has household members (including children) or other close contacts at risk for accidental ingestion or opioid overdose [see Warnings and Precautions (5.4)].
Advise patients and caregivers that naloxone may also be administered for a known or suspected overdose with buprenorphine itself. Higher than normal doses and repeated administration of naloxone may be necessary due to the long duration of action of buprenorphine and its affinity for the mu-opioid receptor [see Overdosage (10)].
Inform patients and caregivers of their options for obtaining naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program) [see Patient Counseling Information (17)].
2.3 Recommended Dosing
Initiation of Treatment following Induction
Patients should first undergo induction and stabilization by initiating a buprenorphine-containing product, delivering the equivalent of 8-24 mg/day of transmucosal buprenorphine for a minimum of 7 days. Dosing and induction with buprenorphine-containing products should be based on instructions in their appropriate product label. One SUBOXONE® (buprenorphine and naloxone) 8 mg/2 mg sublingual tablet provides equivalent buprenorphine exposure to one SUBUTEX® (buprenorphine HCl) 8 mg sublingual tablet or one Bunavail® (buprenorphine and naloxone) 4.2 mg/0.7 mg buccal film or one Zubsolv® (buprenorphine and naloxone) 5.7 mg/1.4 mg sublingual tablet.
The recommended dose of SUBLOCADE following induction is 300 mg monthly for the first two months followed by a maintenance dose of 100 mg monthly.
The maintenance dose may be increased to 300 mg monthly for patients who tolerate the 100 mg dose, but do not demonstrate a satisfactory clinical response, as evidenced by self-reported illicit opioid use or urine drug screens positive for illicit opioid use.
A patient who misses a dose should receive the next dose as soon as possible, with the following dose given no less than 26 days later. Occasional delays in dosing up to 2 weeks are not expected to have a clinically significant impact on treatment effect.
For patients in established treatment of 100 mg monthly, there may be instances when allowance for a two-month dosing interval may be appropriate (e.g., extended travel); in those instances, a single 300 mg dose may be given to cover a two-month period. Thereafter, the 100 mg monthly regimen would resume. Patients should be cautioned that the peak plasma level after injection of a 300 mg dose will be higher than their usual monthly dose and that they may experience sedation or other buprenorphine-related effects.
Transition of Patients Established on Long-term Treatment with Transmucosal Buprenorphine
Patients established on long-term treatment with transmucosal buprenorphine (8-24 mg/day) and whose disease symptoms are controlled may be directly transitioned to SUBLOCADE [see Table 1]. At steady-state, buprenorphine plasma concentrations achieved with 100-mg monthly dosing are contained within the range obtained with transmucosal treatment; peak concentrations with SUBLOCADE may be lower, while average and trough concentrations may be higher. Potential differences in steady state levels need to be taken into consideration when transitioning a patient established on long term treatment with transmucosal buprenorphine to SUBLOCADE [see Clinical Pharmacology (12.3), Table 7].
Table 1 Transition of Patients Established on Long-term Treatment with Transmucosal Buprenorphine Whose Disease Symptoms are Controlled *For patients still experiencing craving or withdrawal symptoms after the initial 300-mg dose, consider giving 300 mg as the second dose
Transmucosal Buprenorphine Doses SUBLOCADE Injection #1 Injection #2 Maintenance Dose 8 – 18 mg/day 300 mg 100 mg* 100 mg 20 – 24 mg/day 300 mg 300 mg 100 mg 2.4 Clinical Supervision
Periodic assessment is necessary to determine effectiveness of the treatment plan and overall patient progress. When evaluating the patient, examine the injection site for signs of infection or evidence of tampering or attempts to remove the depot.
Due to the chronic nature of opioid use disorder, the need for continuing medication-assisted treatment should be re-evaluated periodically. There is no maximum recommended duration of maintenance treatment. For some patients, treatment may continue indefinitely. If considering stopping treatment, the clinical status of the patient should be considered.
If SUBLOCADE is discontinued, its extended-release characteristics should be considered and the patient should be monitored for several months for signs and symptoms of withdrawal and treated appropriately. After steady-state has been achieved (4-6 months), patients discontinuing SUBLOCADE may have detectable plasma and urine levels of buprenorphine for twelve months or longer [see Clinical Pharmacology (12.3)].
2.5 Instructions for Use
IMPORTANT INFORMATION:
- For abdominal subcutaneous injection only. Do not inject intravenously, intramuscularly, or intradermally [see Warnings and Precautions (5.1, 5.6)].
- To be prepared and administered by a healthcare provider only.
- Please read the instructions carefully before handling the product.
- As a universal precaution, always wear gloves.
- Remove SUBLOCADE from the refrigerator prior to administration. The product requires at least 15 minutes to reach room temperature. Do not open the foil pouch until the patient has arrived for his or her injection.
- Discard SUBLOCADE if left at room temperature for longer than 12 weeks.
- Do not attach the needle until time of administration.
STEP 1: GETTING READY
Remove the foil pouch and safety needle from the carton. Open the pouch and remove the syringe.
Discard the oxygen absorber pack. It is not needed.
STEP 2: CHECK THE LIQUID CLARITY
Check that the medication does not contain contaminants or particles. SUBLOCADE ranges from colorless to yellow to amber. Variations of color within this range do not affect the potency of the product.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
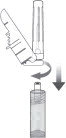
STEP 3: ATTACH THE SAFETY NEEDLE
Remove the cap from the syringe and the safety needle supplied in the carton from its sterile package.
Gently twist the needle clockwise until it is tight and firmly attached.
Do not remove the plastic cover from the needle.
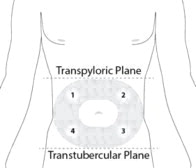
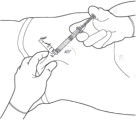
STEP 4: PREPARE THE ABDOMINAL INJECTION SITE
Choose an injection site on the abdomen between the transpyloric and transtubercular planes with adequate subcutaneous tissue that is free of skin conditions (e.g., nodules, lesions, excessive pigment). It is recommended that the patient is in the supine position.
Do not inject into an area where the skin is irritated, reddened, bruised, infected or scarred in any way.
Clean the injection site well with an alcohol swab.
To avoid irritation, rotate injection sites following a pattern similar to the illustration in Figure 4. Record the location of the injection to ensure that a different site is used at the time of the next injection.
STEP 5: REMOVE EXCESS AIR FROM SYRINGE
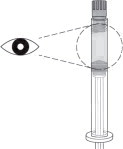
Hold the syringe upright for several seconds to allow air bubbles to rise. Due to the viscous nature of the medication, bubbles will not rise as quickly as those in an aqueous solution.
Remove needle cover and slowly depress the plunger to push out the excess air from the syringe.
- Small bubbles may remain in the medication. Large air gaps, however, can be minimized by pulling back on the plunger rod to pop air bubbles prior to expelling the air very slowly. Air should be expelled very carefully to avoid loss of medication.
If medication is seen at the needle tip, pull back slightly on the plunger to prevent medication spillage.
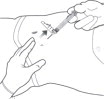
STEP 6: PINCH THE INJECTION SITE
Pinch the skin around the injection area. Be sure to pinch enough skin to accommodate the size of the needle. Lift the adipose tissue from the underlying muscle to prevent accidental intramuscular injection.
STEP 7: INJECT THE MEDICATION
SUBLOCADE is for subcutaneous injection only. Do not inject intravenously, intramuscularly, or intradermally [see Warnings and Precautions (5.1, 5.6)].
Insert needle fully into the abdominal subcutaneous tissue. Actual angle of injection will depend on the amount of subcutaneous tissue.
Use a slow, steady push to inject the medication. Continue pushing until all of the medication is given.
STEP 8: WITHDRAW THE NEEDLE
Withdraw the needle at the same angle used for insertion and release the pinched skin.
Do not rub the injection area after the injection. There may be a small amount of blood or fluid at the injection site; wipe with a cotton ball or gauze before applying a gauze pad or bandage using minimal pressure.
STEP 9: LOCK THE NEEDLE GUARD AND DISCARD THE SYRINGE
Lock the needle guard into place by pushing it against a hard surface such as a table (Figure 9).
Dispose of all syringe components in a secure sharps disposal container.
STEP 10: INSTRUCT THE PATIENT
Advise the patient that they may have a lump for several weeks that will decrease in size over time. Instruct the patient not to rub or massage the injection site and to be aware of the placement of any belts or clothing waistbands.
2.6 Limits on Distribution
SUBLOCADE is subject to a risk evaluation and mitigation strategy (REMS) program that includes, among other elements, a restricted distribution system. The purpose of the restricted distribution system is to ensure that SUBLOCADE is only administered by a health care provider [see Warnings and Precautions (5.2)].
2.7 Removal of the Depot
In the event the depot must be removed, it can be surgically excised under local anesthesia within 14 days of injection. Only the most recently-injected depot can be removed.
The removed depot should be handled with adequate security, accountability, and proper disposal, per facility procedure for a Schedule III drug product and pharmaceutical biohazardous waste, and per applicable federal, state, and local regulations.
The residual plasma concentrations from previous injections will decrease gradually over subsequent months [see Clinical Pharmacology (12.3)].
Patients who have the depot removed should be monitored for signs and symptoms of withdrawal and treated appropriately [see Warnings and Precautions (5.9)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
SUBLOCADE should not be administered to patients who have been shown to be hypersensitive to buprenorphine or any component of the ATRIGEL® delivery system [see Warnings and Precautions (5.11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Serious Harm or Death With Intravenous Administration
Intravenous injection presents significant risk of serious harm or death as SUBLOCADE forms a solid mass upon contact with body fluids. Occlusion, local tissue damage, and thrombo-embolic events, including life threatening pulmonary emboli, could result if administered intravenously [see Warnings and Precautions (5.2), Drug Abuse and Dependence (9.2)]. Do not administer intravenously, intramuscularly, or intradermally [see Warnings and Precautions (5.6)].
5.2 SUBLOCADE Risk Evaluation and Mitigation Strategy (REMS) Program
SUBLOCADE is available only through a restricted program called the SUBLOCADE REMS Program because of the risk of serious harm or death that could result from intravenous self-administration. The goal of the REMS is to mitigate serious harm or death that could result from intravenous self-administration by ensuring that healthcare settings and pharmacies are certified and only dispense SUBLOCADE directly to a healthcare provider for administration by a healthcare provider.
Notable requirements of the SUBLOCADE REMS Program include the following:
- Healthcare Settings and Pharmacies that order and dispense SUBLOCADE must be certified in the SUBLOCADE REMS Program.
- Certified Healthcare Settings and Pharmacies must establish processes and procedures to verify SUBLOCADE is provided directly to a healthcare provider for administration by a healthcare provider, and the drug is not dispensed to the patient.
- Certified Healthcare Settings and Pharmacies must not distribute, transfer, loan, or sell SUBLOCADE.
Further information is available at www.SublocadeREMS.com or call 1-866-258-3905.
5.3 Addiction, Abuse, and Misuse
SUBLOCADE contains buprenorphine, a Schedule III controlled substance that can be abused in a manner similar to other opioids. Buprenorphine is sought by people with opioid use disorder and is subject to criminal diversion. Monitor all patients for progression of opioid use disorder and addictive behaviors [see Drug Abuse and Dependence (9.2)].
5.4 Risk of Life-Threatening Respiratory and Central Nervous System (CNS) Depression
Buprenorphine has been associated with life-threatening respiratory depression and death. Many, but not all, postmarketing reports regarding coma and death involved misuse by self-injection or were associated with the concomitant use of buprenorphine and benzodiazepines or other CNS depressants, including alcohol. Warn patients of the potential danger of self-administration of benzodiazepines or other CNS depressants while under treatment with SUBLOCADE [see Warnings and Precautions (5.5), Drug Interactions (7), Patient Counseling Information (17)].
Use SUBLOCADE with caution in patients with compromised respiratory function (e.g., chronic obstructive pulmonary disease, cor pulmonale, decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression).
Due to its extended-release characteristics, if SUBLOCADE is discontinued as a result of compromised respiratory function, monitor patients for ongoing buprenorphine effects for several months.
Educate patients and caregivers on how to recognize respiratory depression and emphasize the importance of calling 911 or getting emergency medical help right away in the event of a known or suspected overdose [see Patient Counseling Information (17)].
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the opioid dosage using best practices for opioid taper [see Dosage and Administration (2.7)].
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver.
Because patients being treated for opioid use disorder have the potential for relapse, putting them at risk for opioid overdose, strongly consider prescribing naloxone for the emergency treatment of opioid overdose, both when initiating and renewing treatment with SUBLOCADE. Also consider prescribing naloxone if the patient has household members (including children) or other close contacts at risk for accidental ingestion or opioid overdose [see Dosage and Administration (2.2)].
Advise patients and caregivers that naloxone may also be administered for a known or suspected overdose with buprenorphine itself. Higher than normal doses and repeated administration of naloxone may be necessary due to the long duration of action of buprenorphine and its affinity for the mu-opioid receptor [see Overdosage (10)].
Inform patients and caregivers of their options for obtaining naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program).
Educate patients and caregivers on how to recognize respiratory depression and, if naloxone is prescribed, how to treat with naloxone. Emphasize the importance of calling 911 or getting emergency medical help, even if naloxone is administered [see Patient Counseling Information (17)].
5.5 Managing Risks From Concomitant Use of Benzodiazepines or Other CNS Depressants With Buprenorphine
Concomitant use of buprenorphine and benzodiazepines or other CNS depressants increases the risk of adverse reactions including overdose, respiratory depression, and death. Medication-assisted treatment of opioid use disorder, however, should not be categorically denied to patients taking these drugs. Prohibiting or creating barriers to treatment can pose an even greater risk of morbidity and mortality due to the opioid use disorder alone.
As a routine part of orientation to buprenorphine treatment, educate patients about the risks of concomitant use of benzodiazepines, sedatives, opioid analgesics, and alcohol.
Develop strategies to manage use of prescribed or illicit benzodiazepines or other CNS depressants at initiation of buprenorphine treatment, or if it emerges as a concern during treatment. Adjustments to induction procedures and additional monitoring may be required. There is no evidence to support dose limitations or arbitrary caps of buprenorphine as a strategy to address benzodiazepine use in buprenorphine-treated patients. However, if a patient is sedated at the time of buprenorphine dosing, delay or omit the buprenorphine dose if appropriate.
Cessation of benzodiazepines or other CNS depressants is preferred in most cases of concomitant use with buprenorphine. In some cases, monitoring in a higher level of care for taper may be appropriate. In others, gradually tapering a patient off of a prescribed benzodiazepine or other CNS depressant or decreasing to the lowest effective dose may be appropriate.
For patients in buprenorphine treatment, benzodiazepines are not the treatment of choice for anxiety or insomnia. Before co-prescribing benzodiazepines, ensure that patients are appropriately diagnosed and consider alternative medications and non-pharmacologic treatments to address anxiety or insomnia. Ensure that other healthcare providers prescribing benzodiazepines or other CNS depressants are aware of the patient's buprenorphine treatment and coordinate care to minimize the risks associated with concomitant use.
If concomitant use is warranted, strongly consider prescribing naloxone for the emergency treatment of opioid overdose, as is recommended for all patients in buprenorphine treatment for opioid use disorder [see Warnings and Precautions (5.4)].
In addition, take measures to confirm that patients are taking their medications as prescribed and are not diverting or supplementing with illicit drugs. Toxicology screening should test for prescribed and illicit benzodiazepines [see Drug Interactions (7)].
5.6 Risk of Serious Injection Site Reactions
Injection site reactions are most commonly manifested by pain, erythema and pruritus. In some post-marketing case reports injection site reactions have involved abscess, ulceration, and necrosis. Some cases resulted in surgical depot removal, debridement, antibiotic administration, and SUBLOCADE discontinuation. The likelihood of serious injection site reactions may be increased with inadvertent intramuscular or intradermal administration. Carefully review injection technique [see Instructions for Use (2.5)]. Evaluate and treat serious injection site reactions as appropriate.
5.7 Neonatal Opioid Withdrawal Syndrome
Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy, whether that use is medically-authorized or illicit. Unlike opioid withdrawal syndrome in adults, NOWS may be life-threatening if not recognized and treated in the neonate. Healthcare professionals should observe newborns for signs of NOWS and manage accordingly [see Use in Specific Populations (8.1)].
Advise pregnant women receiving opioid addiction treatment with SUBLOCADE of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available [see Use in Specific Populations (8.1)]. This risk should be balanced against the risk of untreated opioid addiction which often results in continued or relapsing illicit opioid use and is associated with poor pregnancy outcomes. Therefore, prescribers should discuss the importance of management of opioid addiction throughout pregnancy.
5.8 Adrenal Insufficiency
Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use. Presentation of adrenal insufficiency may include non-specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
5.9 Risk of Opioid Withdrawal With Abrupt Discontinuation of SUBLOCADE Treatment
Buprenorphine is a partial agonist at the mu-opioid receptor and chronic administration produces physical dependence of the opioid type, characterized by withdrawal signs and symptoms upon abrupt discontinuation. The withdrawal syndrome is milder than that seen with full agonists and may be delayed in onset [see Drug Abuse and Dependence (9.3)].
Withdrawal signs and symptoms were not observed in the month following discontinuation of SUBLOCADE. Considering the long half-life, any withdrawal signs and symptoms that may occur would be expected to be delayed [see Clinical Pharmacology (12.2)]. Model simulations indicate that steady-state buprenorphine plasma concentrations decreased slowly over time following the last injection and remained at therapeutic levels for 2 to 5 months on average, depending on the dosage administered (100 or 300 mg, respectively).
Patients who elect to discontinue treatment with SUBLOCADE should be monitored for withdrawal signs and symptoms. Consider transmucosal buprenorphine if needed to treat withdrawal after discontinuing SUBLOCADE.
5.10 Risk of Hepatitis, Hepatic Events
Cases of cytolytic hepatitis and hepatitis with jaundice have been observed in individuals receiving buprenorphine in clinical trials and through postmarketing adverse event reports. The spectrum of abnormalities ranges from transient asymptomatic elevations in hepatic transaminases to case reports of death, hepatic failure, hepatic necrosis, hepatorenal syndrome, and hepatic encephalopathy. In many cases, the presence of pre-existing liver enzyme abnormalities, infection with hepatitis B or hepatitis C virus, concomitant usage of other potentially hepatotoxic drugs, and ongoing injecting drug use may have played a causative or contributory role. In other cases, insufficient data were available to determine the etiology of the abnormality. Withdrawal of buprenorphine has resulted in amelioration of acute hepatitis in some cases, however, in other cases no dose reduction was necessary. The possibility exists that buprenorphine had a causative or contributory role in the development of the hepatic abnormality in some cases. In one subject in the SUBLOCADE clinical program, surgical removal was followed by improvement in liver enzymes.
Liver function tests, prior to initiation of treatment, are recommended to establish a baseline. Monthly monitoring of liver function during treatment, particularly with 300 mg maintenance dose, is also recommended. An etiological evaluation is recommended when a hepatic adverse event is suspected.
5.11 Hypersensitivity Reactions
Cases of hypersensitivity to buprenorphine-containing products have been reported both in clinical trials and in the postmarketing experience. Cases of bronchospasm, angioneurotic edema, and anaphylactic shock have been reported. The most common signs and symptoms include rashes, hives, and pruritus. A history of hypersensitivity to buprenorphine is a contraindication to the use of SUBLOCADE [see Contraindications (4)].
5.12 Precipitation of Opioid Withdrawal in Patients Dependent on Full Agonist Opioids
Because of the partial opioid agonist properties of buprenorphine, buprenorphine may precipitate opioid withdrawal signs and symptoms in persons who are currently physically dependent on full opioid agonists such as heroin, morphine, or methadone before the effects of the full opioid agonist have subsided. Verify that patients have tolerated and are dose adjusted on transmucosal buprenorphine before subcutaneously injecting SUBLOCADE.
5.13 Risks Associated With Treatment of Emergent Acute Pain
While on SUBLOCADE, situations may arise where patients need acute pain management, or may require anesthesia. Treat patients receiving SUBLOCADE with a non-opioid analgesic whenever possible. Patients requiring opioid therapy for analgesia may be treated with a high-affinity full opioid analgesic under the supervision of a physician, with particular attention to respiratory function. Higher doses may be required for analgesic effect. Therefore, a higher potential for toxicity exists with opioid administration. If opioid therapy is required as part of anesthesia, patients should be continuously monitored in an anesthesia care setting by persons not involved in the conduct of the surgical or diagnostic procedure. The opioid therapy should be provided by individuals specifically trained in the use of anesthetic drugs and the management of the respiratory effects of potent opioids, specifically the establishment and maintenance of a patent airway and assisted ventilation.
Advise patients of the importance of instructing their family members, in the event of emergency, to inform the treating healthcare provider or emergency room staff that the patient is physically dependent on an opioid and that the patient is being treated with SUBLOCADE [see Patient Counseling Information (17)].
The above guidance should also be considered for any patient who has been treated with SUBLOCADE within the last 6 months.
5.14 Use in Opioid Naïve Patients
There have been reported deaths of opioid naïve individuals who received a 2 mg dose of buprenorphine as a sublingual tablet. SUBLOCADE is not appropriate for use in opioid naïve patients.
5.15 Use in Patients With Impaired Hepatic Function
In a pharmacokinetic study with transmucosal buprenorphine, buprenorphine plasma levels were found to be higher and the half-life was found to be longer in subjects with moderate and severe hepatic impairment, but not in subjects with mild hepatic impairment. The effect of hepatic impairment on the pharmacokinetics of SUBLOCADE has not been studied.
Because of the long-acting nature of the product, adjustments to dosages of SUBLOCADE are not rapidly reflected in plasma buprenorphine levels. Because buprenorphine levels cannot be rapidly decreased, patients with pre-existing moderate to severe hepatic impairment are not candidates for treatment with SUBLOCADE.
Patients who develop moderate to severe hepatic impairment while being treated with SUBLOCADE should be monitored for several months for signs and symptoms of toxicity or overdose caused by increased levels of buprenorphine [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
5.16 QTc Prolongation
Thorough QT studies with buprenorphine products have demonstrated QT prolongation ≤ 15 msec. This QTc prolongation effect does not appear to be mediated by hERG channels. Based on these two findings, buprenorphine is unlikely to be pro-arrhythmic when used alone in patients without risk factors. The risk of combining buprenorphine with other QT-prolonging agents is not known.
Consider these observations in clinical decisions when prescribing SUBLOCADE to patients with risk factors such as hypokalemia, bradycardia, recent conversion from atrial fibrillation, congestive heart failure, digitalis therapy, baseline QT prolongation, subclinical long-QT syndrome, or severe hypomagnesemia.
5.17 Impairment of Ability to Drive or Operate Machinery
SUBLOCADE may impair the mental or physical abilities required for the performance of potentially dangerous tasks such as driving a car or operating machinery especially during the first few days following treatment and dose adjustment. Buprenorphine plasma levels accumulate during the first two months and are maintained with the 100 mg maintenance dose; further accumulation occurs with the 300 mg maintenance dose, which achieves steady-state after the fourth monthly injection. Caution patients about driving or operating hazardous machinery until they are reasonably certain that SUBLOCADE does not adversely affect their ability to engage in such activities.
5.18 Orthostatic Hypotension
Buprenorphine may produce orthostatic hypotension in ambulatory patients.
5.19 Elevation of Cerebrospinal Fluid Pressure
Buprenorphine may elevate cerebrospinal fluid pressure and should be used with caution in patients with head injury, intracranial lesions, and other circumstances when cerebrospinal pressure may be increased. Buprenorphine can produce miosis and changes in the level of consciousness that may interfere with patient evaluation.
5.20 Elevation of Intracholedochal Pressure
Buprenorphine has been shown to increase intracholedochal pressure, as do other opioids, and thus should be administered with caution to patients with dysfunction of the biliary tract.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Addiction, Abuse, and Misuse [see Warnings and Precautions (5.3)]
- Respiratory and CNS Depression [see Warnings and Precautions (5.4)]
- Neonatal Opioid Withdrawal Syndrome [see Warnings and Precautions (5.7)]
- Adrenal Insufficiency [see Warnings and Precautions (5.8)]
- Opioid Withdrawal [see Warnings and Precautions (5.9, 5.12)]
- Hepatitis, Hepatic Events [see Warnings and Precautions (5.10)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.11)]
- Orthostatic Hypotension [see Warnings and Precautions (5.18)]
- Elevation of Cerebrospinal Fluid Pressure [see Warnings and Precautions (5.19)]
- Elevation of Intracholedochal Pressure [see Warnings and Precautions (5.20)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
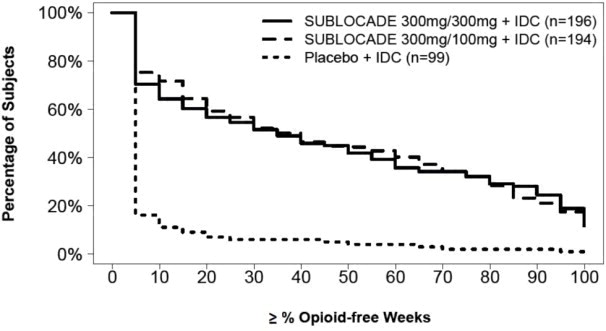
The safety of SUBLOCADE was evaluated in 848 opioid-dependent subjects (see Table 2). In these studies, there was a total of 557 subjects who received at least 6 monthly SC injections of SUBLOCADE and 138 subjects who received 12 monthly SC injections. Adverse events led to premature discontinuation in 4% of the group receiving SUBLOCADE compared with 2% in the placebo group (13-0001, NCT02357901).
In the Phase 3 open-label study (13-0003, NCT02510014), adverse events leading to drug dose reductions were reported in 7.3% of subjects receiving SUBLOCADE.
Table 2 Total Subjects Exposed to SUBLOCADE *Not included in total subjects exposed to SUBLOCADE
† FLEX = 300 mg initial dose with an option to receive either 100 mg or 300 mg for subsequent dosing per clinician's discretion
‡ = Not included in total unique subjects exposed to SUBLOCADE, already accounted for in Study 13-0001 section of table
Study 13-0001 (NCT02357901)
Up to 6 InjectionsStudy 13-0003 (NCT02510014) Total Subjects Exposed To
SUBLOCADERoll-Over
Up to 6 InjectionsDe-Novo
Up to 12 InjectionsSUBLOCADE 300/100 mg SUBLOCADE 300/300 mg Placebo From SUBLOCADE 300/100 mg To
SUBLOCADE 300/Flex†From SUBLOCADE 300/300 mg To
SUBLOCADE 300/Flex†From Placebo
To
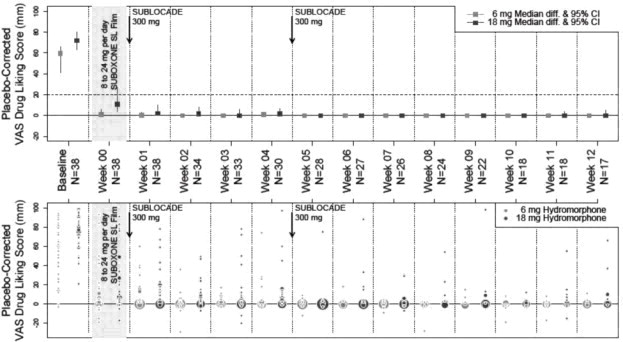
SUBLOCADE 300/Flex†SUBLOCADE 300/Flex N = 203 N = 201 N = 100* N = 112‡ N = 113‡ N = 32 N = 412 N = 848 Table 3 shows the non-injection site-related adverse reactions (ADRs) for the groups receiving SUBLOCADE 300/300 mg (6 doses of 300 mg SC injections) 300/100 mg (300 mg SC injections for the first two doses followed by 4 doses of 100 mg SC injections) and placebo (volume-matched ATRIGEL® delivery system subcutaneous injections) reported following administration in the 6 month, double-blind, placebo-controlled study. The systemic safety profile for SUBLOCADE, given by a healthcare provider in clinical trials, was consistent with the known safety profile of transmucosal buprenorphine. Common adverse reactions associated with buprenorphine included constipation, nausea, vomiting, abnormal liver enzymes, headache, sedation and somnolence. Dose dependent hepatic effects observed in the Phase 3, double-blind study (13-0001, NCT02357901) included the incidence of ALT more than 3 times the upper limit of normal (> 3 × ULN) in 12.4%, 5.4%, and 4.0% of the SUBLOCADE 300/300-mg, SUBLOCADE 300/100-mg, and placebo groups, respectively. The incidence of AST > 3 × ULN was 11.4%, 7.9%, and 1.0%, respectively. Adverse drug reactions [by MedDRA Preferred Terms (PT)] reported in at least 2% of subjects receiving SUBLOCADE are grouped by System Organ Class (SOC).
Table 3 Adverse Reactions for Phase 3 Double-Blind Study: ≥ 2% of Subjects Receiving SUBLOCADE System Organ Class
Preferred TermPLACEBO SUBLOCADE
300/100 mgSUBLOCADE
300/300 mgCount (%) Count (%) Count (%) *There were no cases of serious liver injury attributed to study drug.
Total N = 100 N = 203 N = 201 Gastrointestinal disorders 12 (12%) 51 (25.1%) 45 (22.4%) Constipation 0 19 (9.4) 16 (8) Nausea 5 (5) 18 (8.9) 16 (8) Vomiting 4 (4) 19 (9.4) 11 (5.5) General disorders and administration site conditions 17 (17%) 40 (19.7%) 49 (24.4%) Fatigue 3 (3) 8 (3.9) 12 (6) Investigations* 2 (2%) 21 (10.3%) 19 (9.5%) Alanine aminotransferase increased (ALT) 0 2 (1) 10 (5) Aspartate aminotransferase increased (AST) 0 7 (3.4) 9 (4.5) Blood creatine phosphokinase increased (CPK) 1 (1) 11 (5.4) 5 (2.5) Gamma-glutamyl transferase increased (GGT) 1 (1) 6 (3) 8 (4) Nervous system disorders 7 (7%) 35 (17.2%) 25 (12.4%) Headache 6 (6) 19 (9.4) 17 (8.5) Sedation 0 7 (3.4) 3 (1.5) Dizziness 2 (2) 5 (2.5) 3 (1.5) Somnolence 0 10 (4.9) 4 (2) Table 4 shows the injection site-related adverse events reported by ≥ 2 subjects in the Phase 3 studies. Most injection site adverse drug reactions (ADRs) were of mild to moderate severity, with one report of severe injection site pruritus. None of the injection site reactions were serious. One reaction, an injection site ulcer, led to study treatment discontinuation.
Table 4 Injection Site Adverse Drug Reactions Reported by ≥ 2 Subjects in the Phase 3 Studies *Patients received SUBOXONE film for a run-in period before they switched to SUBLOCADE injection.
Preferred term, n (%) 13-0001 (Ph3DB) 13-0003 (Ph3OL) All
Phase 3*Roll–over De-novo SUBLOCADE
300/300
(N = 201)SUBLOCADE 300/100
(N = 203)Placebo
(N = 100)SUBLOCADE 300 →
SUBLOCADE
300/Flex
(N = 113)SUBLOCADE 100 →
SUBLOCADE
300/Flex
(N = 112)Placebo →
SUBLOCADE 300/Flex
(N = 32)SUBLOCADE 300/Flex
(N = 412)Total
SUBLOCADE
(N = 848)Subjects with any injection site reactions 38 (18.9%) 28 (13.8%) 9 (9.0%) 6 (5.3%) 13 (11.6%) 2 (6.3%) 61 (14.8%) 140 (16.5%) Injection site pain 12 (6.0%) 10 (4.9%) 3 (3.0%) 4 (3.5%) 2 (1.8%) 2 (6.3%) 33 (8.0%) 61 (7.2%) Injection site pruritus 19 (9.5%) 13 (6.4%) 4 (4.0%) 2 (1.8%) 6 (5.4%) 1 (3.1%) 17 (4.1%) 56 (6.6%) Injection site erythema 6 (3.0%) 9 (4.4%) 0 1 (0.9%) 4 (3.6%) 0 21 (5.1%) 40 (4.7%) Injection site induration 2 (1.0%) 2 (1.0%) 0 0 1 (0.9%) 0 7 (1.7%) 12 (1.4%) Injection site bruising 2 (1.0%) 2 (1.0%) 0 0 0 0 2 (0.5%) 6 (0.7%) Injection site swelling 1 (0.5%) 2 (1.0%) 0 1 (0.9%) 1 (0.9%) 0 1 (0.2%) 6 (0.7%) Injection site discomfort 1 (0.5%) 1 (0.5%) 0 0 0 0 3 (0.7%) 5 (0.6%) Injection site reaction 1 (0.5%) 0 0 0 3 (2.7%) 0 1 (0.2%) 5 (0.6%) Injection site cellulitis 0 1 (0.5%) 0 0 0 0 2 (0.5%) 3 (0.4%) Injection site infection 1 (0.5%) 0 1 (1.0%) 0 0 0 2 (0.5%) 3 (0.4%) Longer-term experience
In an interim analysis of the ongoing open-label long-term safety study (13-0003), safety was evaluated for up to 12 injections over the course of a year (see Table 2). Adverse events were reported for 432 of 669 subjects during the treatment period. The overall adverse event profile was similar to the double-blind trial described above.
6.2 Postmarketing Experience
The most frequently reported systemic postmarketing adverse event observed with buprenorphine sublingual tablets was drug misuse or abuse. The most frequently reported systemic postmarketing adverse event with buprenorphine/naloxone sublingual tablets and film was peripheral edema.
The following adverse reactions have been identified during post-approval use of buprenorphine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Serotonin syndrome: Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of opioids with serotonergic drugs.
Adrenal insufficiency: Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use.
Anaphylaxis: Anaphylaxis has been reported with ingredients contained in SUBLOCADE.
Androgen deficiency: Cases of androgen deficiency have occurred with chronic use of opioids [see Clinical Pharmacology (12.2)].
Hypoglycemia: Cases of hypoglycemia have been reported in patients taking opioids. Most reports were in patients with at least one predisposing risk factor (e.g., diabetes).
-
7 DRUG INTERACTIONS
Table 5 includes clinically significant drug interactions with SUBLOCADE.
Table 5 Clinically Significant Drug Interactions Benzodiazepines and Other Central Nervous System (CNS) Depressants Clinical Impact: Due to additive pharmacologic effects, the concomitant use of benzodiazepines or other CNS depressants, including alcohol, increases the risk of respiratory depression, profound sedation, coma, and death. Intervention: Cessation of benzodiazepines or other CNS depressants is preferred in most cases of concomitant use. In some cases, monitoring in a higher level of care for taper may be appropriate. In others, gradually tapering a patient off of a prescribed benzodiazepine or other CNS depressant or decreasing to the lowest effective dose may be appropriate. Similarly, cessation of other CNS depressants is preferred when possible.
Before co-prescribing benzodiazepines for anxiety or insomnia, ensure that patients are appropriately diagnosed and consider alternative medications and non-pharmacologic treatments [see Warnings and Precautions (5.4, 5.5)].
If concomitant use is warranted, strongly consider prescribing naloxone for the emergency treatment of opioid overdose, as is recommended for all patients in treatment for opioid use disorder [see Warnings and Precautions (5.4)].Examples: Alcohol, benzodiazepines and other sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, and other opioids. Inhibitors of CYP3A4 Clinical Impact: The effects of co-administered CYP3A4 inhibitors on buprenorphine exposure in subjects treated with SUBLOCADE have not been studied and the effects may be dependent on the route of administration; however, such interactions have been established in studies using transmucosal buprenorphine. Buprenorphine is metabolized to norbuprenorphine primarily by CYP3A4; therefore, potential interactions may occur when SUBLOCADE is given concurrently with agents that affect CYP3A4 activity.
The concomitant use of sublingual buprenorphine and CYP3A4 inhibitors (e.g., ketoconazole) can increase the plasma concentration of buprenorphine, resulting in increased or prolonged opioid effects.Intervention: Patients who transfer to SUBLOCADE treatment from a regimen of transmucosal buprenorphine used concomitantly with CYP3A4 inhibitors [e.g., azole antifungals such as ketoconazole, macrolide antibiotics such as erythromycin, and HIV protease inhibitors (e.g., ritonavir, indinavir, and saquinavir)] should be monitored to ensure that the plasma buprenorphine level provided by SUBLOCADE is adequate. If patients already on SUBLOCADE require newly-initiated treatment with CYP3A4 inhibitors, the patients should be monitored for signs and symptoms of over-medication. Within 2 weeks of SUBLOCADE administration, if signs and symptoms of buprenorphine toxicity or overdose occur but the concomitant medication cannot be reduced or discontinued, it may be necessary to remove the depot and treat the patient with a formulation of buprenorphine that permits dose adjustments. Conversely, if a patient has been stabilized on SUBLOCADE in the setting of concomitant medication that is a CYP3A4 inhibitor, and the concomitant medication is discontinued, the patient should be monitored for withdrawal. If the dose of SUBLOCADE is not adequate in the absence of the concomitant medication, that patient should be transitioned back to a formulation of buprenorphine that permits dose adjustments. Examples: Macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g., ketoconazole), protease inhibitors (e.g., ritonavir) CYP3A4 Inducers Clinical Impact: The effects of co-administered CYP3A4 inducers on buprenorphine exposure in subjects treated with SUBLOCADE have not been studied.
Buprenorphine is metabolized to norbuprenorphine primarily by CYP3A4; therefore, potential interactions may occur when SUBLOCADE is given concurrently with agents that affect CYP3A4 activity.
CYP3A4 inducers may induce the metabolism of buprenorphine and, therefore, may cause increased clearance of the drug which could lead to a decrease in buprenorphine plasma concentrations, lack of efficacy or, possibly, development of an abstinence syndrome.Intervention: Patients who transfer to SUBLOCADE treatment from a regimen of transmucosal buprenorphine used concomitantly with CYP3A4 inducers should be monitored to ensure that the plasma buprenorphine level provided by SUBLOCADE is adequate. If patients already on SUBLOCADE require newly-initiated treatment with CYP3A4 inducers, the patients should be monitored for withdrawal. If the dose of SUBLOCADE is not adequate in the absence of the concomitant medication, and the concomitant medication cannot be reduced or discontinued, that patient should be transitioned back to a formulation of buprenorphine that permits dose adjustments. Conversely, if a patient has been stabilized on SUBLOCADE in the setting of concomitant medication that is a CYP3A4 inducer, and the concomitant medication is discontinued, the patient should be monitored for signs and symptoms of over-medication. Within 2 weeks of SUBLOCADE administration, if the dose provided by SUBLOCADE is excessive in the absence of the concomitant inducer, it may be necessary to remove the SUBLOCADE and treat the patient with a formulation of buprenorphine that permits dose adjustments [see Clinical Pharmacology (12.3)]. Examples: Rifampin, carbamazepine, phenytoin, phenobarbital Antiretrovirals: Non-nucleoside reverse transcriptase inhibitors (NNRTIs) Clinical Impact: Non-nucleoside reverse transcriptase inhibitors (NNRTIs) are metabolized principally by CYP3A4. Efavirenz, nevirapine, and etravirine are known CYP3A inducers, whereas delavirdine is a CYP3A inhibitor. Significant pharmacokinetic interactions between NNRTIs (e.g., efavirenz and delavirdine) and sublingual buprenorphine have been shown in clinical studies, but these pharmacokinetic interactions did not result in any significant pharmacodynamic effects. Intervention: Patients who are on chronic treatment with SUBLOCADE should be monitored for increase or decrease in therapeutic effects if NNRTIs are added to their treatment regimen. Examples: Efavirenz, nevirapine, etravirine, delavirdine Antiretrovirals: Protease inhibitors (PIs) Clinical Impact: Studies have shown some antiretroviral protease inhibitors (PIs) with CYP3A4 inhibitory activity (nelfinavir, lopinavir/ritonavir, ritonavir) have little effect on sublingual buprenorphine pharmacokinetic and no significant pharmacodynamic effects. Other PIs with CYP3A4 inhibitory activity (atazanavir and atazanavir/ritonavir) resulted in elevated levels of buprenorphine and norbuprenorphine after sublingual administration, and patients in one study reported increased sedation. Symptoms of opioid excess have been found in postmarketing reports of patients receiving sublingual buprenorphine and atazanavir with and without ritonavir concomitantly. Intervention: If treatment with atazanavir with and without ritonavir must be initiated in a patient already treated with SUBLOCADE, the patient should be monitored for signs and symptoms of over-medication. It may be necessary to remove the depot and treat the patient with a sublingual buprenorphine product that permits rapid dose adjustments. Examples: Atazanavir, ritonavir Antiretrovirals: Nucleoside reverse transcriptase inhibitors (NRTIs) Clinical Impact: Nucleoside reverse transcriptase inhibitors (NRTIs) do not appear to induce or inhibit the P450 enzyme pathway, thus no interactions with buprenorphine are expected. Intervention: None Serotonergic Drugs Clinical Impact: The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system has resulted in serotonin syndrome. Intervention: If concomitant use is warranted, carefully monitor the patient for signs and symptoms of serotonin syndrome, particularly during treatment initiation, and during dose adjustment of the serotonergic drug. Examples: Selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that affect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), certain muscle relaxants (i.e., cyclobenzaprine, metaxalone), monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue). Monoamine Oxidase Inhibitors (MAOIs) Clinical Impact: MAOI interactions with opioids may manifest as serotonin syndrome or opioid toxicity (e.g., respiratory depression, coma). Intervention: The use of SUBLOCADE is not recommended for patients taking MAOIs or within 14 days of stopping such treatment. Examples: Phenelzine, tranylcypromine, linezolid Muscle Relaxants Clinical Impact: Buprenorphine may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression. Intervention: Monitor patients receiving muscle relaxants and SUBLOCADE for signs of respiratory depression that may be greater than otherwise expected and decrease the dosage of the muscle relaxant as necessary. Due to the risk of respiratory depression with concomitant use of skeletal muscle relaxants and opioids, strongly consider prescribing naloxone for the emergency treatment of opioid overdose [see Dosage and Administration (2.2), Warnings and Precautions (5.4, 5.5)]. Diuretics Clinical Impact: Opioids can reduce the efficacy of diuretics by inducing the release of antidiuretic hormone. Intervention: Monitor patients for signs of diminished diuresis and/or effects on blood pressure and increase the dosage of the diuretic as needed. Anticholinergic Drugs Clinical Impact: The concomitant use of anticholinergic drugs may increase the risk of urinary retention and/or severe constipation, which may lead to paralytic ileus. Intervention: Monitor patients for signs of urinary retention or reduced gastric motility when SUBLOCADE is used concomitantly with anticholinergic drugs. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The data on use of buprenorphine, the active ingredient in SUBLOCADE, in pregnancy, are limited; however, these data do not indicate an increased risk of major malformations specifically due to buprenorphine exposure. There are limited data from randomized clinical trials in women maintained on buprenorphine that were not designed appropriately to assess the risk of major malformations [see Human Data].
Observational studies have reported congenital malformations among buprenorphine‐exposed pregnancies, but were also not designed appropriately to assess the risk of congenital malformations specifically due to buprenorphine exposure [see Human Data].
In animal reproduction studies with SUBLOCADE, SUBLOCADE administered subcutaneously to pregnant rats and rabbits during the period of organogenesis at a buprenorphine dose equivalent to 38 and 15 times, respectively, the maximum recommended human dose (MRHD) of 300 mg caused embryolethality, which appeared to be attributable primarily to the SUBLOCADE vehicle (ATRIGEL® delivery system). In addition, reduced fetal body weights, increased visceral malformations and skeletal malformations were observed in rats and rabbits at buprenorphine doses equivalent to 38 and 15 times, respectively, the MRHD. These effects were also observed with the ATRIGEL® delivery system alone, but the skeletal and visceral malformations in rat appear at least partially attributable to buprenorphine [see Animal Data]. Based on animal data, advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
SUBLOCADE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Clinical Considerations
Disease-associated maternal and embryo-fetal risk
Untreated opioid addiction in pregnancy is associated with adverse obstetrical outcomes such as low birth weight, preterm birth, and fetal death. In addition, untreated opioid addiction often results in continued or relapsing illicit opioid use.
Fetal/neonatal adverse reactions
Neonatal opioid withdrawal syndrome may occur in newborn infants of mothers who are receiving treatment with SUBLOCADE.
Neonatal opioid withdrawal syndrome presents as irritability, hyperactivity and abnormal sleep pattern, high pitched cry, tremor, vomiting, diarrhea, and/or failure to gain weight. Signs of neonatal withdrawal usually occur in the first days after birth. The duration and severity of neonatal opioid withdrawal syndrome may vary. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly [see Warnings and Precautions (5.7)].
Labor or Delivery
Opioid-dependent women on buprenorphine maintenance therapy may require additional analgesia during labor. As with all opioids, use of buprenorphine prior to delivery may result in respiratory depression in the newborn. Closely monitor neonates for signs of respiratory depression. An opioid antagonist such as naloxone should be available for reversal of opioid induced respiratory depression in the neonate.
Data
Human Data
Studies have been conducted to evaluate neonatal outcomes in women exposed to buprenorphine during pregnancy. Limited data on malformations from trials, observational studies, case series, and case reports on buprenorphine use in pregnancy do not indicate an increased risk of major malformations specifically due to buprenorphine. Pregnancy in an opioid dependent woman poses challenges to treating physicians and potential hazards for the fetus including control of illicit drug, nicotine and alcohol use, infections, premature birth, abortion, low birth weight, toxaemia, third trimester bleeding, malpresentation, puerperal morbidity, fetal distress, meconium aspiration, narcotic withdrawal, postnatal growth deficiency, microcephaly, (neuro-) developmental disorders and increased neonatal mortality.
A multicenter, double-blind, double-dummy, flexible-dose study in 175 pregnant women [Maternal Opioid Treatment: Human Experimental Research (MOTHER)] was conducted to study outcomes in neonates born to mothers using methadone or buprenorphine, including the number of neonates requiring treatment for NOWS, the Peak NOWS score, the total amount of morphine needed to treat NOWS, the length of hospital stay for neonates, and neonatal head circumference. The authors found that 18% of pregnant women in the methadone group and 33% in the buprenorphine group discontinued treatment over the course of the pregnancy. They reported no significant difference in the incidence of NOWS, but in the prenatally buprenorphine-exposed condition, the duration of treatment for NOWS was shorter, duration of hospital stays were shorter and the amount of morphine required was significantly less; however, methodological concerns limit the conclusions that may be made.
Animal Data
In an embryofetal development study in rats, SUBLOCADE administered subcutaneously to pregnant animals before mating and again on Gestation Day (GD) 7 during the period of organogenesis resulted in increased post-implantation loss, which correlated with higher mean number of resorptions and decreased number of viable fetuses per litter, and decreased mean fetal body weights at 900 mg/kg (approximately 38 times the maximum recommended human dose [MRHD] of 300 mg of SUBLOCADE on an AUC basis); however, similar effects were observed with an equivalent level of ATRIGEL® delivery system alone, indicating they may be attributable to the vehicle. Dose-related increases in incidences of skeletal malformations of the head and visceral malformations were observed with SUBLOCADE with significant changes at 900 mg/kg (approximately 38 times the MRHD on an AUC basis). Although similar effects were observed with equivalent levels of ATRIGEL® delivery system, the incidence of skeletal malformations, primarily skull malformations, was higher in the SUBLOCADE groups suggesting that buprenorphine contributed to the increased incidence. Based on these results, the NOAEL for developmental toxicity was approximately 15 times the MRHD on an AUC basis.
In an embryofetal development study in rabbits, administration of a single subcutaneous injection of SUBLOCADE to pregnant animals on GD 7 during the period of organogenesis resulted an increased litter incidence of skeletal malformations at 155 mg/kg (approximately 7 times the MRHD on an AUC basis), which appear to be buprenorphine-related adverse effects. There was also an increased litter incidence of external malformations, visceral, and skeletal malformations and variations at 390 mg/kg SUBLOCADE (approximately 15 times the MRHD on an AUC basis); however, similar effects were observed with an equivalent level of the ATRIGEL® delivery system, indicating they may be attributable to the vehicle. In addition, increased post-implantation loss, which correlated with increased mean number of resorptions and decreased mean number of viable fetuses, and decreased fetal body weights were observed at 390 mg/kg (approximately 15 times the MRHD on an AUC basis); however, similar findings were also observed with an equivalent level of the ATRIGEL® delivery system alone. Based on these results, the NOAEL for developmental toxicity for SUBLOCADE was 78 mg/kg (approximately 2 times the MRHD on an AUC basis).
In a pre- and postnatal development study in rats, SUBLOCADE was administered subcutaneously to pregnant animals once during implantation (on GD 7) and once during weaning (on Lactation Day 7). There were no adverse effects on offspring survival, sexual maturation, behavioral assessment, or reproductive performance at up to 300 mg/kg (approximately 15 times the MRHD on an AUC basis).
8.2 Lactation
Risk Summary
Based on two studies in 13 lactating women maintained on buprenorphine treatment, buprenorphine and its metabolite norbuprenorphine are present in low levels in human milk and infant urine. Available data have not shown adverse reactions in breastfed infants. Caution should be exercised when SUBLOCADE is administered to a nursing woman. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for SUBLOCADE and any potential adverse effects on the breastfed child from the drug or from the underlying maternal condition.
Clinical Considerations
Advise breastfeeding women taking buprenorphine products to monitor the infant for increased drowsiness and breathing difficulties.
Data
Data were consistent from two studies (N=13) of breastfeeding infants whose mothers were maintained on sublingual doses of buprenorphine ranging from 2.4 to 24 mg/day, showing that the infants were exposed to less than 1% of the maternal daily dose.
In a study of six lactating women who were taking a median sublingual buprenorphine dose of 0.29 mg/kg/day 5 to 8 days after delivery, breast milk provided a median infant dose of 0.42 mcg/kg/day of buprenorphine and 0.33 mcg/kg/day of norbuprenorphine, equal to 0.2% and 0.12%, respectively, of the maternal weight-adjusted dose (relative dose/kg (%) of norbuprenorphine was calculated from the assumption that buprenorphine and norbuprenorphine are equipotent).
Data from a study of seven lactating women who were taking a median sublingual buprenorphine dose of 7 mg/day an average of 1.12 months after delivery indicated that the mean milk concentrations (Cavg) of buprenorphine and norbuprenorphine were 3.65 mcg/L and 1.94 mcg/L respectively. Based on the study data, and assuming milk consumption of 150 mL/kg/day, an exclusively breastfed infant would receive an estimated mean absolute infant dose (AID) of 0.55 mcg/kg/day of buprenorphine and 0.29 mcg/kg/day of norbuprenorphine, or a mean relative infant dose (RID) of 0.38% and 0.18%, respectively, of the maternal weight-adjusted dose.
8.3 Females and Males of Reproductive Potential
Human Data
Chronic use of opioids may cause reduced fertility in females and males of reproductive potential. It is not known whether these effects on fertility are reversible [see Adverse Reactions (6.2)].
Animal Data
Infertility
Male
Male fertility may be reduced based on animal data demonstrating adverse effects of SUBLOCADE on sperm parameters [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of SUBLOCADE have not been established in pediatric patients.
8.5 Geriatric Use
Clinical studies of SUBLOCADE did not include sufficient numbers of subjects aged 65 and over to determine whether they responded differently than younger subjects. Other reported clinical experience with buprenorphine has not identified differences in responses between geriatric and younger patients.
Due to possible decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy in geriatric patients, the decision to prescribe SUBLOCADE should be made cautiously in individuals 65 years of age or older and these patients should be monitored for signs and symptoms of toxicity or overdose.
8.6 Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of SUBLOCADE has not been studied.
The effect of hepatic impairment on the pharmacokinetics of sublingual buprenorphine has been evaluated in a pharmacokinetic study. While no clinically significant changes have been observed in subjects with mild hepatic impairment, the plasma levels have been shown to be higher and half-life values have been shown to be longer for buprenorphine in subjects with moderate and severe hepatic impairment.
Because of the long-acting nature of the product, adjustments to dosages of SUBLOCADE are not rapidly reflected in plasma buprenorphine levels. Because buprenorphine levels cannot be rapidly adjusted, patients with pre-existing moderate to severe hepatic impairment are not candidates for treatment with SUBLOCADE.
Patients who develop moderate to severe hepatic impairment while being treated with SUBLOCADE should be monitored for signs and symptoms of toxicity or overdose caused by increased levels of buprenorphine. If signs and symptoms of toxicity or overdose occur within 2 weeks of SUBLOCADE administration, removal of the depot may be required [see Dosage and Administration (2.7), Warnings and Precautions (5.10), Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
SUBLOCADE contains buprenorphine, a Schedule III substance under the Controlled Substances Act.
9.2 Abuse
SUBLOCADE contains buprenorphine, a Schedule III controlled substance that can be abused similar to other opioids. Patients who continue to misuse, abuse, or divert buprenorphine products or other opioids should be provided with or referred for more intensive and structured treatment. Abuse of buprenorphine poses a risk of overdose and death. This risk is increased with the abuse of buprenorphine and alcohol and other substances, especially benzodiazepines.
SUBLOCADE is distributed through a restricted distribution system, which is intended to prevent the direct distribution to a patient. SUBLOCADE should only be dispensed directly to a healthcare provider for administration by a healthcare provider. It is supplied in prefilled syringes and is intended for administration only by subcutaneous injection by a healthcare provider. The entire contents of the prefilled syringe should be administered. After administration, a small amount (approximately 0.1 mL) of SUBLOCADE will remain in the needle and syringe and should be properly disposed of [see How Supplied/Storage and Handling (16)].
SUBLOCADE is injected as a liquid, and the subsequent precipitation of the poly (DL-lactide-co-glycolide) polymer creates a solid depot which contains buprenorphine. After initial formation of the depot, buprenorphine is released via diffusion from, and the biodegradation of, the depot. Clinical monitoring for evidence at the injection site of tampering or attempting to remove the depot should be ongoing throughout treatment. No accounts of subjects removing or attempting to remove the depot after administration of SUBLOCADE were reported in premarketing studies.
9.3 Dependence
Buprenorphine is a partial agonist at the mu-opioid receptor and chronic administration produces physical dependence of the opioid type, characterized by moderate withdrawal signs and symptoms upon abrupt discontinuation. The withdrawal syndrome is typically milder than seen with full agonists and may be delayed in onset [see Warnings and Precautions (5.12)].
Due to the long-acting nature of SUBLOCADE, withdrawal signs and symptoms may not be evident immediately following the discontinuation of treatment.
Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy [see Warnings and Precautions (5.7)].
-
10 OVERDOSAGE
Clinical Presentation
The manifestations of acute buprenorphine overdose include pinpoint pupils, sedation, hypotension, hypoglycemia, respiratory depression, and death.
Treatment of Overdose
In the event of overdose, the respiratory and cardiac status of the patient should be monitored carefully. When respiratory or cardiac functions are depressed, primary attention should be given to the re-establishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. Oxygen, IV fluids, vasopressors, and other supportive measures should be considered as indicated. Naloxone may be of value for the management of buprenorphine overdose. Higher than normal doses and repeated administration may be necessary.
Clinicians should consider the potential role and contribution of buprenorphine, other opioids, and other CNS depressant drugs in a patient's clinical presentation. Clinical data are limited with regards to the possible surgical removal of the depot. Two cases of surgical removal were reported in premarketing clinical studies.
-
11 DESCRIPTION
SUBLOCADE (buprenorphine extended-release) injection is a clear, viscous, colorless to yellow to amber, sterile solution for subcutaneous injection only. It is designed to deliver buprenorphine at a controlled rate over a one month period.
The active ingredient in SUBLOCADE is buprenorphine free base, a mu-opioid receptor partial agonist and a kappa-opioid receptor antagonist.
Buprenorphine is dissolved in the ATRIGEL® delivery system at 18% by weight.
The ATRIGEL® delivery system is a biodegradable 50:50 poly(DL-lactide-co-glycolide) polymer and a biocompatible solvent, N-methyl-2-pyrrolidone (NMP).
SUBLOCADE is provided in dosage strengths of 100 mg and 300 mg. Table 6 presents the delivered amounts of the raw materials and the approximate delivered volume for the two dosage strengths.
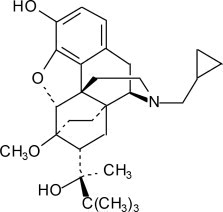
Table 6 Amounts of Raw Materials and Delivered Volume for the Dosage Strengths Raw Materials in SUBLOCADE 100 mg Dosage 300 mg Dosage Buprenorphine 100 mg 300 mg Poly(DL-lactide-co-glycolide) 178 mg 533 mg N-methyl-2-pyrrolidone 278 mg 833 mg Approximate Delivered Volume 0.5 mL 1.5 mL The molecular weight of buprenorphine free base is 467.6, and its molecular formula is C29H41NO4. Chemically, buprenorphine is (2S)-2-[17-(Cyclopropylmethyl)-4,5α-epoxy-3-hydroxy-6-methoxy-6α,14-ethano-14α-morphinan-7α-yl]-3,3-dimethylbutan-2-ol. The structural formula is:
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
SUBLOCADE Injection contains buprenorphine. Buprenorphine is a partial agonist at the mu- opioid receptor and an antagonist at the kappa-opioid receptor.
12.2 Pharmacodynamics
Mu-Opioid Receptor Occupancy and Association With Opioid Blockade
In a Positron Emission Tomography (PET) study with SUBLOCADE in 2 subjects (one subject receiving 200 mg subcutaneous injections and one subject receiving 300 mg subcutaneous injections) with opioid use disorder, 75 to 92% occupancy of the mu-opioid receptors in the brain was maintained for 28 days following the last dose under steady-state conditions.
The opioid blockade study evaluated the blockade of subjective opioid effects, pharmacokinetics (PK) and safety of subcutaneous injections of SUBLOCADE. Stabilization doses of SL buprenorphine prior to injection of SUBLOCADE failed to provide full blockade of subjective effects of hydromorphone 18 mg IM. After SUBLOCADE injections at Weeks 0 and 4, on average, subjective effects of both 6 mg and 18 mg doses of hydromorphone were blocked; however, wide variability was seen across subjects. Complete blockade continued throughout the 8 weeks of observation that followed the 2nd SUBLOCADE injection [see Clinical Studies (14.1)].
Figure 10 illustrates the relationship between buprenorphine plasma level and drug liking after 18 mg hydromorphone IM.
Figure 10 Drug Liking VAS vs. Plasma Buprenorphine Concentration Following 18 mg Hydromorphone Challenges
Exposure-response relationships were assessed for illicit opioid use, based on urine samples negative for illicit opioids combined with self-reports negative for illicit opioid use, and withdrawal symptoms using data obtained from 489 opioid dependent patients in the double-blind Phase 3 Study (13-0001).
The observed plateau for maximal response was reached at buprenorphine plasma concentrations of approximately 2-3 ng/mL for illicit opioid use and 4 ng/mL for opioid withdrawal symptoms.
Population PK/PD modeling indicated that patients using opioids by the injectable route at baseline may require higher buprenorphine exposure compared to patients not using opioids by the injectable route at baseline.
Cardiac Electrophysiology
Serial ECGs were collected following a single dose and at steady-state to evaluate the effect of SUBLOCADE on the QT interval in five clinical studies including the Phase 3 study. In a Phase 3 study, seven patients had an increase from baseline QTc greater than 60 msec at any time [2/203 patients (1.0%) in the 300 mg/100 mg group and 5/201 patients (2.0%) in the 300 mg/300 mg group] and one patient in the 300 mg/300 mg group was found to have a QTc greater than 500 msec. These QTc findings were all sporadic and transient and none led to aberrant ventricular rhythm. Review of ECG and adverse event data provided no evidence for syncope, seizure, or ventricular tachycardia or fibrillation.
Physiological Effects
Buprenorphine in IV (2, 4, 8, 12 and 16 mg) and sublingual (12 mg) doses have been administered to opioid-experienced subjects who were not physically dependent to examine cardiovascular, respiratory, and subjective effects at doses comparable to those used for treatment of opioid dependence. Compared to placebo, there were no statistically significant differences among any of the treatment conditions for blood pressure, heart rate, respiratory rate, O2 saturation, or skin temperature across time. Systolic BP was higher in the 8 mg group than placebo (3 hour AUC values). Minimum and maximum effects were similar across all treatments. Subjects remained responsive to low voice and responded to computer prompts. Some subjects showed irritability, but no other changes were observed. The respiratory effects of sublingual buprenorphine were compared with the effects of methadone in a double-blind, parallel group, dose ranging comparison of single doses of buprenorphine sublingual solution (1, 2, 4, 8, 16, or 32 mg) and oral methadone (15, 30, 45, or 60 mg) in non-dependent, opioid-experienced volunteers. In this study, hypoventilation not requiring medical intervention was reported more frequently after buprenorphine doses of 4 mg and higher than after methadone. Both drugs decreased O2 saturation to the same degree.
In clinical studies conducted with SUBLOCADE at doses ranging from 50 to 300 mg, no incidences of temperature elevations, or clinically significant lowering of oxygen saturation were observed.
Androgen Deficiency
Chronic use of opioids may influence the hypothalamic-pituitary-gonadal axis, leading to androgen deficiency that may manifest as low libido, impotence, erectile dysfunction, amenorrhea, or infertility. The causal role of opioids in the clinical syndrome of hypogonadism is unknown because the various medical, physical, lifestyle, and psychological stressors that may influence gonadal hormone levels have not been adequately controlled for in studies conducted to date. Patients presenting with symptoms of androgen deficiency should undergo laboratory evaluation.
Pharmacodynamic Interaction with Fentanyl
An open-label, cross-over study was conducted in 8 opioid-tolerant subjects to assess the ability of intravenous buprenorphine to prevent respiratory depression associated with high doses of fentanyl administered in a clinical setting. Opioid-tolerant subjects were medically stable and taking oral morphine equivalents of ≥ 90 mg daily with no other CNS depressant use. Buprenorphine infusions at three dose levels and placebo infusions were administered, followed by up to four doses of fentanyl. The three intravenous buprenorphine dose levels were designated as low (0.25 mg/70kg bolus + 0.1 mg/70kg/hr), mid (0.5 mg/70kg bolus + 0.2 mg/70kg/hr), and high (1.25 mg/70kg bolus + 0.5 mg/70kg/hr). The low, mid, and high buprenorphine dose levels produced average plasma concentrations (from 2 hours to 6 hours after infusion onset) of 1.06 ng/mL, 2.26 ng/mL, and 6.04 ng/mL, respectively.
Escalating intravenous fentanyl boluses of 0.25, 0.35, 0.50 and 0.70 mg/70 kg (up to a maximum cumulative dose of 1.8 mg/70 kg) were administered over 90 seconds at + 2hr, + 3hr, + 4hr and + 5hr after the start of intravenous infusion of buprenorphine or placebo. Apnea events after each fentanyl bolus are shown in Figure 11. Four of the 8 subjects in the placebo infusion groups discontinued prior to the fourth fentanyl bolus because of apnea (2 after the second bolus and 2 after the third bolus); 3 of the remaining 4 subjects experienced apnea after the fourth fentanyl dose. All 8 subjects in the buprenorphine infusion groups completed the fentanyl boluses and 2 of the 8 experienced apnea.
Figure 11 Occurrence of Apnea Events After Fentanyl Doses in a Pharmacodynamic Interaction Study
There were 2 opioid-tolerant subjects in the low intravenous buprenorphine (BUP) group (Arm A), 3 in the mid BUP group (Arm B), and 3 in the high BUP group (Arm C). The numbers in parentheses represent the geometric mean of buprenorphine plasma concentrations in each group from 2 hours to 6 hours after infusion onset. The escalating fentanyl doses 1 to 4 were 0.25, 0.35, 0.50 and 0.70 mg/70 kg body weight. The same subject in the low BUP group had apnea after the third and fourth fentanyl boluses.
12.3 Pharmacokinetics
Absorption
The pharmacokinetics (PK) of buprenorphine following subcutaneous injection of SUBLOCADE was evaluated in subjects with opioid use disorder after single doses (50 mg to 200 mg) and repeated doses (50 to 300 mg) separated by 28 days for up to 12 injections.
After SUBLOCADE injection, an initial buprenorphine peak was observed and the median Tmax occurred at 24 hours after injection. After the initial buprenorphine peak, the plasma buprenorphine concentrations decreased slowly to a plateau. Steady-state was achieved at 4-6 months.
Mean buprenorphine plasma concentrations levels for Cavg, Cmax and Ctrough at steady-state are presented in Table 7 for SUBLOCADE in comparison to daily transmucosal buprenorphine.
Table 7 Comparison of Steady-state Buprenorphine Plasma Exposure Between Daily Transmucosal Buprenorphine and Once Monthly SUBLOCADE at Trough (Ctrough), Average (Cavg) and Peak (Cmax) Levels (Geometric Mean (CV%)) Pharmacokinetic parameters Transmucosal Buprenorphine SUBLOCADE 8 mg 12 mg 16 mg 24 mg 100 mg 300 mg Cavg,ss (ng/mL) 1.37
(40)1.79
(40)2.16
(40)2.84
(40)2.87
(32)6.32
(32)Cmax,ss (ng/mL) 4.27
(45)5.60
(45)6.77
(45)8.86
(45)5.10
(33)11.81
(35)Ctrough,ss (ng/mL) 0.66
(63)0.87
(63)1.04
(61)1.37
(62)2.46
(40)5.47
(39)Distribution
Buprenorphine is approximately 96% protein bound, primarily to alpha and beta globulin.
Elimination
Buprenorphine is metabolized and eliminated in urine and feces. The apparent terminal plasma half-life of buprenorphine following subcutaneous injection of SUBLOCADE ranged between 43 to 60 days as a result of the slow release of buprenorphine from the subcutaneous depot.
A study assessing buprenorphine exposure 22 to 38 months following the last SUBLOCADE injection indicated that buprenorphine could potentially be detected in plasma and urine over that time period. Concentrations in urine were more variable than in plasma and generally higher depending on the test used. Hence, it is expected that buprenorphine will be detected in patients for a longer time in urine than in plasma.
Metabolism
Buprenorphine is metabolized to its major metabolite, norbuprenorphine, primarily by CYP3A4. Norbuprenorphine can further undergo glucuronidation. Norbuprenorphine has been found to bind opioid receptors in vitro; however, it has not been studied clinically for opioid-like activity. Norbuprenorphine steady-state plasma concentrations in humans after subcutaneous injection of SUBLOCADE are low compared to buprenorphine (AUC norbuprenorphine/buprenorphine ratio of 0.20 to 0.40).
Excretion
A mass balance study of buprenorphine administered by IV infusion in humans showed complete recovery of radiolabel in urine (30%) and feces (69%) collected up to 11 days after dosing. Almost all of the dose was accounted for in terms of buprenorphine, norbuprenorphine, and two unidentified buprenorphine metabolites. In urine, most of buprenorphine and norbuprenorphine were conjugated (buprenorphine: 1% free and 9.4% conjugated; norbuprenorphine: 2.7% free and 11% conjugated). In feces, almost all of the buprenorphine and norbuprenorphine were free (buprenorphine: 33% free and 5% conjugated; norbuprenorphine: 21% free and 2% conjugated).
Drug Interaction Studies
CYP3A4 Inhibitors and Inducers
The effects of co-administered CYP3A4 inhibitors and inducers on buprenorphine exposure in subjects treated with SUBLOCADE have not been studied; however, such interactions have been established in studies using transmucosal buprenorphine. The effects of buprenorphine may be dependent on the route of administration.
Buprenorphine is metabolized to norbuprenorphine primarily by cytochrome CYP3A4; therefore, potential interactions may occur when SUBLOCADE is given concurrently with agents that affect CYP3A4 activity. The effects of co-administered CYP3A4 inducers or inhibitors have been established in studies using transmucosal buprenorphine. Patients who transfer to SUBLOCADE treatment from a regimen of transmucosal buprenorphine used concomitantly with CYP3A4 inhibitors (e.g., ketoconazole), macrolide antibiotics (e.g., erythromycin), or HIV protease inhibitors, or CYP3A4 inducer (e.g., phenobarbital, carbamazepine, phenytoin, rifampicin) should be monitored to ensure that the plasma buprenorphine level provided by SUBLOCADE is adequate and not excessive [see Drug Interactions (7)].
Buprenorphine has been found to be a CYP2D6 and CYP3A4 inhibitor and its major metabolite, norbuprenorphine, has been found to be a moderate CYP2D6 inhibitor in in vitro studies employing human liver microsomes. However, the plasma concentrations of buprenorphine and norbuprenorphine resulting from therapeutic SUBLOCADE doses are not expected to significantly affect metabolism of other co-medications.
Specific Populations
Based on population pharmacokinetic analyses, age, sex and race do not have a clinically meaningful effect on PK of SUBLOCADE.
Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of SUBLOCADE has not been studied. However, the effect of hepatic impairment on the PK of buprenorphine has been evaluated in a study using 2 mg/0.5 mg buprenorphine/naloxone sublingual tablet in subjects with various degrees of hepatic impairment as indicated by Child-Pugh criteria. While no clinically relevant changes were observed in subjects with mild hepatic impairment, buprenorphine plasma exposure was increased by 64% and 181% in subjects with moderate and severe hepatic impairment, respectively, compared to healthy subjects [see Use in Specific Populations (8.6)].
Renal Impairment
The effect of renal impairment on the pharmacokinetics of SUBLOCADE has not been studied. Clinical studies of SUBLOCADE did not include subjects with severe renal impairment.
Less than 1% is excreted as unchanged buprenorphine in urine following IV buprenorphine administration. No differences in buprenorphine pharmacokinetics were observed between 9 dialysis-dependent and 6 normal patients following IV administration of 0.3 mg buprenorphine [see Use in Specific Populations (8.7)].
Population PK analyses indicated no notable relationship between creatinine clearance and steady-state buprenorphine plasma concentrations.
HCV infection
In subjects with HCV infection but no sign of hepatic impairment, the changes in the mean Cmax, AUC0-last, and half-life values of buprenorphine were not clinically significant in comparison to healthy subjects without HCV infection. No dose adjustment is needed in patients with HCV infection.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Long-term studies in animals performed to evaluate carcinogenic potential of SUBLOCADE have not been conducted. However, the carcinogenic potential of the active drug substance in SUBLOCADE, buprenorphine, has been evaluated in Sprague-Dawley rats and CD-1 mice.
In the carcinogenicity study conducted in Sprague-Dawley rats, buprenorphine was administered in the diet at doses of 0.6, 5.5, and 56 mg/kg/day (approximately 0.5, 5, and 50 times the recommended human monthly subcutaneous dose of 300 mg of buprenorphine) for 27 months. A statistically significant dose-related increase in Leydig cell tumors occurred. In an 86 week study in CD-1 mice, buprenorphine was not carcinogenic at dietary doses up to 100 mg/kg/day (approximately 45 times the recommended human monthly subcutaneous dose of 300 mg of buprenorphine).
N-methyl-2-pyrrolidone [NMP], an excipient in SUBLOCADE, produced an increase in hepatocellular adenomas and carcinomas in male and female mice at 6 and 8 times the maximum daily dose (MDD) of NMP via SUBLOCADE. The clinical significance of these findings is unclear. No tumors were noted at 1 and 1.3 times the MDD. In 2-year inhalation and dietary studies in rats, NMP did not result in evidence of carcinogenicity.
Mutagenicity
No evidence of mutagenic potential for subcutaneous SUBLOCADE was found in in vivo subcutaneous micronucleus test using rats' marrow.
Mutagenic potential for buprenorphine was studied in a series of tests utilizing gene, chromosome, and DNA interactions in both prokaryotic and eukaryotic systems. Results were negative in yeast (S. cerevisiae) for recombinant, gene convertant, or forward mutations; negative in Bacillus subtilis “rec” assay, negative for clastogenicity in CHO cells, Chinese hamster bone marrow and spermatogonia cells, and negative in the mouse lymphoma L5178Y assay.
Results were equivocal in the Ames test: negative in studies in two laboratories, but positive for frame shift mutation at a high dose (5 mg/plate) in a third study. Results were positive in the Green-Tweets (E. coli) survival test, positive in a DNA synthesis inhibition (DSI) test with testicular tissue from mice, for both in vivo and in vitro incorporation of [3H]thymidine, and positive in unscheduled DNA synthesis (UDS) test using testicular cells from mice.
Impairment of Fertility
In a fertility study in rats, SUBLOCADE or ATRIGEL® was administered every 28 days for 3 months (males) or once before mating and once on GD 7; female mating, fertility, and fecundity indices were unaffected by the subcutaneous administration of SUBLOCADE up to 900 mg/kg buprenorphine (approximately 38 times the maximum recommended human dose [MRHD] of 300 mg on an AUC basis). However, higher mean post-implantation loss was observed with SUBLOCADE at 900 mg/kg buprenorphine and at an equivalent level of ATRIGEL® alone, which correlated with higher mean number of resorptions and reduced mean number of viable fetuses/litter size. Mean gravid uterine weight and mean final body weight were lower with SUBLOCADE at 900 mg/kg buprenorphine and an equivalent level of ATRIGEL® alone, and correlated with higher mean number of resorptions and lower fetal body weights. The NOAEL for female fertility was 900 mg/kg and the NOAEL for female-mediated developmental parameters was 600 mg/kg (approximately 25 times the MRHD on an AUC basis).
Male fertility and reproduction indices were lower as evidenced by abnormal sperm parameters (low motility, low mean number of sperm, and higher percentage of abnormal sperm) with SUBLOCADE at 600 mg/kg and with an equivalent level of ATRIGEL®. The NOAEL for male fertility parameters, including sperm analysis, and male-mediated developmental parameters was 300 mg/kg (approximately 32 times the MRHD on an AUC basis).
-
14 CLINICAL STUDIES
The key studies from the SUBLOCADE clinical development program that support its use in moderate to severe OUD are a Phase 3 double-blind efficacy and safety study (13-0001, NCT02357901) and an opioid blockade study (13-0002, NCT02044094).
14.1 Study 13-0002, NCT02044094
The opioid blockade study evaluated the blockade of subjective opioid effects, PK and safety of subcutaneous injections of SUBLOCADE in 39 subjects with OUD (not treatment-seeking).
The peak (Emax) effect of “Drug Liking” Visual Analog Scale (VAS) measurement after challenge with IM injections of 6 mg and 18 mg hydromorphone (HM) was not inferior (i.e., shown to be not substantially more likeable) compared to the Emax of “Drug Liking” VAS measured after challenge with placebo (at weeks 1 through 4 following the first injection of 300 mg SUBLOCADE). The noninferiority (NI) margin, the largest difference allowed for the 6 or 18 mg HM VAS to exceed the placebo VAS (the maximum VAS recorded following IM injection of 0 mg HM) before being considered significant, was set at 20. Based on comparison to the historical response to opioid agonists in unblocked subjects, a difference of less than 20 points (on a unipolar scale) between the mean maximum response to hydromorphone and the mean maximum placebo response for the same challenge was considered to indicate near-complete blockade.
All 12 weeks of the treatment period demonstrated blockade for both 6 mg and 18 mg following SUBLOCADE injections. However, wide variation can be seen in isolated measurements from individual subjects, shown in the figure below. For comparison, stabilization doses of SL buprenorphine in Week 0 failed to provide full blockade to 18 mg of HM. Complete blockade continued throughout the 8 weeks of observation that followed the 2nd SUBLOCADE injection.
Figure 12 Median (95% Confidence Interval) of Placebo-Corrected Drug-Liking Scores by Hydromorphone Dose and by Week
- Key to Figure: The grey shaded area indicates the period where subjects were stabilized with 8 to 24 mg/day sublingual (SL) buprenorphine; the two vertical arrows represent treatment injections of SUBLOCADE, with 300 mg of buprenorphine.
- The light grey and dark grey squares represent the median Emax drug-liking scores, placebo-corrected (VAS drug liking for that week's 0 mg dose subtracted) during the hydromorphone challenge of 6 and 18 mg, respectively. This median Placebo-Corrected Emax is shown by treatment week, together with its 95% confidence interval (CI; vertical line). In some cases, 95% CI are not visible as the median was equal to the confidence limit. The horizontal line at 20 mm delineates the non-inferiority margin for opioid blockade. Next to median estimates, individual data are summarized by circles, the area of which is proportional to the number of subjects at that location.
- The X axis shows how many weeks following injection #1 that each weeks' Placebo-Corrected Drug-Liking Score was measured. Beneath that treatment week indicator, is the number of subjects (N) who provided those VAS measurements for all three challenges with placebo, 6 and 18 mg hydromorphone.
14.2 Study 13-0001, NCT02357901
The efficacy of SUBLOCADE for the treatment of opioid use disorder was evaluated in a Phase 3, 24- week, randomized, double-blind, placebo-controlled, multicenter trial in treatment-seeking patients who met the DSM-5 criteria for moderate or severe opioid use disorder. Patients were randomized to one of following dosing regimens: 6 once-monthly 300 mg doses, 2 once-monthly 300 mg doses followed by 4 once-monthly 100 mg doses, or 6 once-monthly subcutaneous injections of placebo. All doses were administered by a physician or suitably qualified designee and were separated by 28 ± 2 days. In addition to study medication, all subjects received manual-guided psychosocial support at least once a week (Individual Drug Counseling = IDC).
Prior to the first dose, treatment was initiated with SUBOXONE® (buprenorphine/naloxone) sublingual film (SUBOXONE SL Film); doses were adjusted from 8/2 mg to 24/6 mg per day over a period of 7-14 days. Patients were randomized to SUBLOCADE injection or placebo after cravings and withdrawal symptoms were clinically controlled. After randomization, supplemental dosing with SUBOXONE SL Film was not permitted during the study.
Efficacy was evaluated over Weeks 5 through 24 based on weekly urine drug screens combined with self-reported use of illicit opioid use. A “grace period” was applied for Weeks 1 through 4 to allow patients to stabilize in treatment. During this period, opioid use, if it occurred, was not considered in the analysis. Missing urine drug screen samples and/or self-reports during Weeks 5-24 were counted as positive for illicit opioids.
A total of 504 patients were randomized 4:4:1:1 [203 subjects in the 300 mg/100 mg group, 201 patients in the 300 mg/300 mg group and 100 patients in the placebo group (2 groups of volume-matched placebo)]. Patient demographics and baseline characteristics are provided in Table 8.
Table 8 Patient Demographics and Baseline Characteristics SUBLOCADE 300/100 mg % SUBLOCADE 300/300 mg % Placebo % Mean Age (years) 40.4 39.3 39.2 Sex Male 66.0 67.3 64.6 Female 34.0 32.7 35.4 Race or Ethnicity White 68.0 71.4 77.8 Black or African American 28.9 27.6 20.2 Hispanic or Latino 6.2 9.2 10.1 Substance Use At Screening Opioid Use - Injectable Route 43.3 40.8 50.5 Tobacco 91.8 92.3 92.9 Alcohol 78.4 79.1 80.8 Drug Use History Cannabinoids 54.6 47.4 52.5 Cocaine 47.4 39.8 42.4 Amphetamine/Methamphetamine 25.3 14.8 19.2 Medical History Depression 14.4 11.2 13.1 Anxiety 9.3 9.7 10.1 Back Pain 14.9 16.3 13.1 Based on the cumulative distribution function (CDF) of the percentage of urine samples negative for illicit opioids combined with self-reports negative for illicit opioid use collected from Week 5 through Week 24 (Table 9), regardless of dose, SUBLOCADE was superior to the placebo group with statistical significance. The proportion of patients achieving treatment success (defined as patients with ≥ 80% opioid-free weeks) was statistically significantly higher in both groups receiving SUBLOCADE compared to the placebo group (28.4% [300 mg/100 mg], 29.1% [300 mg/300mg], 2% [placebo]).
For various percentages of opioid-free weeks, Table 9 shows the fraction of patients achieving that criterion. The table is cumulative, so that a patient whose percent of opioid-free weeks is, for example, 50%, is also included at every level of opioid-free week percentage below 50%. Missing values and values after premature discontinuation were considered positive.
Figure 13 Subjects Achieving Varying Percentages of Opioid-Free Weeks
Table 9 Cumulative Distribution Function of Percentage of Opioid-Free Weeks Number (%) of Subjects SUBLOCADE SUBLOCADE 300 mg/100 mg + IDC 300 mg/300 mg + IDC Placebo + IDC Percentage Opioid-Free Weeks (N = 194) (N = 196) (N = 99) ≥ 0% 194 (100.0) 196 (100.0) 99 (100.0) ≥ 10% 139 (71.6) 126 (64.3) 11 (11.1) ≥ 20% 115 (59.3) 111 (56.6) 7 (7.1) ≥ 30% 101 (52.1) 101 (51.5) 6 (6.1) ≥ 40% 90 (46.4) 90 (45.9) 6 (6.1) ≥ 50% 86 (44.3) 82 (41.8) 4 (4.0) ≥ 60% 78 (40.2) 70 (35.7) 4 (4.0) ≥ 70% 66 (34.0) 67 (34.2) 2 (2.0) ≥ 80% 55 (28.4) 57 (29.1) 2 (2.0) ≥ 90% 41 (21.1) 48 (24.5) 2 (2.0) = 100% 25 (13) 23 (12) 1 (1.0) -
16 HOW SUPPLIED/STORAGE AND HANDLING
SUBLOCADE is available as a sterile, clear, viscous, colorless to yellow to amber solution in a single dose, prefilled syringe with safety needle.
SUBLOCADE, 100 mg/0.5 mL – NDC 12496-0100-1
SUBLOCADE, 300 mg/1.5 mL – NDC 12496-0300-1
Storage and Handling
Store refrigerated at 2°C to 8°C (35.6°F to 46.4°F).
Once outside the refrigerator this product may be stored in its original packaging at room temperature, 15°C to 30°C (59°F to 86°F), for up to 12 weeks prior to administration. Discard SUBLOCADE if left at room temperature for longer than 12 weeks.
SUBLOCADE is a Schedule III drug product. Handle with adequate security and accountability. After administration, syringes should be properly disposed, per facility procedure for a Schedule III drug product, and per applicable federal, state, and local regulations.
Rx Only.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
SUBLOCADE Risk Evaluation and Mitigation Strategy (REMS)
Advise patients that because of the risk of serious harm or death due to intravenous self-administration, SUBLOCADE is available only through a restricted program called the SUBLOCADE REMS Program. Healthcare settings and pharmacies are certified and only dispense SUBLOCADE directly to a healthcare provider for administration by healthcare providers [see Warnings and Precautions (5.2)].
Life Threatening Respiratory Depression
Educate patients and caregivers on how to recognize respiratory depression and emphasize the importance of calling 911 or getting emergency medical help right away in the event of a known or suspected overdose [see Warnings and Precautions 5.4)].
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Because patients being treated for opioid use disorder are at risk for relapse, discuss the importance of having access to naloxone with the patient and caregiver. Also discuss the importance of having access to naloxone if there are household members (including children) or other close contacts at risk for accidental ingestion or opioid overdose.
Inform patients and caregivers of the options for obtaining naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program).
Educate patients and caregivers on how to recognize the signs and symptoms of an opioid overdose.
Explain to patients and caregivers that naloxone's effects are temporary, and that they must call 911 or get emergency medical help right away in all cases of known or suspected opioid overdose, even if naloxone is administered. Repeat administration may be necessary, particularly for overdose involving buprenorphine, because naloxone is often not effective at the doses available for patient access [see Dosage and Administration (2.2), Warnings and Precautions (5.4), Overdosage (10)].
If naloxone is prescribed, also advise patients and caregivers:
- How to treat with naloxone in the event of an opioid overdose
- To tell family and friends about their naloxone and to keep it in a place where family and friends can easily access it in an emergency
- To read the Patient Information (or other educational material) that will come with their naloxone. Emphasize the importance of doing this before an opioid emergency happens, so the patient and caregiver will know what to do
Interaction With Benzodiazepines and Other CNS Depressants
Inform patients and caregivers that potentially fatal additive effects may occur if SUBLOCADE is used with benzodiazepines or other CNS depressants, including alcohol. Counsel patients that such medications should not be used concomitantly unless supervised by a healthcare provider [see Warnings and Precautions (5.4, 5.5), Drug Interactions (7)].
Serotonin Syndrome
Inform patients that SUBLOCADE could cause a rare but potentially life-threatening condition resulting from concomitant administration of serotonergic drugs. Warn patients of the symptoms of serotonin syndrome and to seek medical attention right away if symptoms develop. Instruct patients to inform their healthcare providers if they are taking, or plan to take serotonergic medications [see Drug Interactions (7)].
Adrenal Insufficiency
Inform patients that SUBLOCADE could cause adrenal insufficiency, a potentially life-threatening condition. Adrenal insufficiency may present with non-specific symptoms and signs such as nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. Advise patients to seek medical attention if they experience a constellation of these symptoms [see Warnings and Precautions (5.8)].
Anaphylaxis
Inform patients that anaphylaxis has been reported with buprenorphine. Advise patients how to recognize such a reaction and when to seek medical attention [see Warnings and Precautions (5.11)].
Driving or Operating Heavy Machinery
Caution patients that SUBLOCADE may impair the mental or physical abilities required for the performance of potentially dangerous tasks such as driving or operating hazardous machinery. Instruct patients not to drive or operate hazardous machinery until they are reasonably certain that SUBLOCADE does not adversely affect their ability to engage in such activities [see Warnings and Precautions (5.17)].
Dependence and Withdrawal
Inform patients that SUBLOCADE can cause drug dependence and that withdrawal signs and symptoms may occur when the medication is discontinued [see Warnings and Precautions (5.9, 5.12)].
Orthostatic Hypotension
Inform patients that, like other opioids, SUBLOCADE may produce orthostatic hypotension in ambulatory individuals [see Warnings and Precautions (5.18)].
Long Duration of Action
Inform patients that they may have detectable levels of buprenorphine for a prolonged period of time after treatment with SUBLOCADE. Considerations of drug-drug interactions, buprenorphine effects, and analgesia may continue to be relevant for several months after the last injection [see Clinical Pharmacology (12.3)].
Drug Interactions
Instruct patients to inform their healthcare providers of any other prescription medications, over the-counter medications, or herbal preparations that are prescribed or currently being used [see Drug Interactions (7)].
Pregnancy
Neonatal Opioid Withdrawal Syndrome
Advise women that if they are pregnant while being treated with SUBLOCADE, the baby may have signs of withdrawal at birth and that withdrawal is treatable [see Warnings and Precautions (5.6), Use in Specific Populations (8.1)].
Embryofetal Toxicity
Advise women of childbearing potential who become pregnant or are planning to become pregnant to consult their healthcare provider regarding the possible effects of using SUBLOCADE during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Warn patients that buprenorphine passes into breast milk. Advise the nursing mother taking buprenorphine to monitor the infant for increased drowsiness and breathing difficulties [see Use in Specific Populations (8.2)].
Infertility
Inform patients that chronic use of opioids may cause reduced fertility. It is not known whether these effects on fertility are reversible [see Use in Specific Populations (8.3), Clinical Pharmacology (12.2)].
Emergency Analgesia
Patients should be advised to instruct their family members to, in the event of emergency, inform the treating healthcare provider or emergency room staff that the patient is physically dependent on an opioid and that the patient is being treated with SUBLOCADE [see Warnings and Precautions (5.13)].
Clinical Monitoring
Tell your patients to seek emergency attention if they have signs or symptoms of respiratory or CNS depression or overdose [see Warnings and Precautions (5.4, 5.5)].
Tell your patients not to tamper with or try to remove their depot [see Dosage and Administration (2.7)].
© 2023, Indivior UK Limited. All Rights Reserved.
SUBLOCADE® and SUBOXONE® are registered trademarks of Indivior UK Limited.
Manufactured for Indivior Inc., North Chesterfield VA, 23235
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Issued: 08/2022
MEDICATION GUIDE
SUBLOCADE (SUB-lo-kade) (buprenorphine extended-release) injection, for subcutaneous use, (CIII)What is the most important information I should know about SUBLOCADE?
- Because of the serious risk of potential harm or death from self-injecting SUBLOCADE into a vein (intravenously), it is only available through a restricted program called the SUBLOCADE REMS Program.
- SUBLOCADE is not available in retail pharmacies.
- Your SUBLOCADE injection will only be given to you by a certified healthcare provider.
- SUBLOCADE contains a medicine called buprenorphine. Buprenorphine is an opioid that can cause serious and life-threatening breathing problems, especially if you take or use certain other medicines or drugs.
- Talk to your healthcare provider about naloxone. Naloxone is a medicine that is available to patients for the emergency treatment of an opioid overdose. If naloxone is given, you must call 911 or get emergency medical help right away to treat overdose or accidental use of an opioid.
- SUBLOCADE may cause serious and life-threatening breathing problems. Get emergency help right away if you:
- feel faint
- feel dizzy
- are confused
- Feel sleepy or uncoordinated
- have blurred vision
- have slurred speech
- are breathing slower than normal
- cannot think well or clearly
Do not take certain medicines during treatment with SUBLOCADE. Taking other opioid medicines, benzodiazepines, alcohol, or other central nervous system depressants (including street drugs) while on SUBLOCADE can cause severe drowsiness, decreased awareness, breathing problems, coma, and death.
- In an emergency, have family members tell emergency department staff that you are physically dependent on an opioid and are being treated with SUBLOCADE.
- You may have detectable levels of SUBLOCADE in your body for a long period after stopping treatment with SUBLOCADE.
What is SUBLOCADE?
SUBLOCADE is a prescription medicine used to treat adults with moderate to severe addiction (dependence) to opioid drugs (prescription or illegal) who:
have received treatment with an oral transmucosal (used under the tongue or inside the cheek) buprenorphine-containing medicine for 7 days and
are taking a dose that controls withdrawal symptoms for at least seven days.
SUBLOCADE is part of a complete treatment plan that should include counseling.
Who should not take SUBLOCADE?
Do not use SUBLOCADE if you are allergic to buprenorphine or any ingredient in the prefilled syringe (ATRIGEL® delivery system). See the end of this Medication Guide for a list of ingredients in SUBLOCADE.Before starting SUBLOCADE, tell your healthcare provider about all your medical conditions, including if you have: - trouble breathing or lung problems
- a curve in your spine that affects your breathing
- Addison's disease
- an enlarged prostate (men)
- problems urinating
- liver, kidney, or gallbladder problems
- alcoholism
- a head injury or brain problem
- mental health problems
- adrenal gland or thyroid gland problems
Tell your healthcare provider if you are:
- pregnant or plan to become pregnant. If you receive SUBLOCADE while pregnant, your baby may have symptoms of opioid withdrawal at birth that could be life-threatening if not recognized and treated. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
-
breastfeeding or plan to breastfeed. SUBLOCADE can pass into your breast milk and harm your baby. Talk to your healthcare provider about the best way to feed your baby during treatment with SUBLOCADE. Monitor your baby for increased drowsiness and breathing problems if you breastfeed during treatment with SUBLOCADE.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. How will I receive SUBLOCADE?
- You will receive SUBLOCADE by your healthcare provider as an injection just under the skin (subcutaneous) of your stomach (abdomen). You will receive SUBLOCADE monthly (with at least 26 days between doses).
- SUBLOCADE is injected as a liquid. After the injection, SUBLOCADE changes to a solid form called a depot. The depot may be seen or felt as a small bump under your skin at the injection site on your abdomen for several weeks. The depot will get smaller over time.
- Do not try to remove the depot.
- Do not rub or massage the injection site.
- Try not to let belts or clothing waistbands rub against the injection site.
- If you miss a dose of SUBLOCADE, see your healthcare provider to get your SUBLOCADE injection as soon as possible.
What should I avoid while being treated with SUBLOCADE?
- Do not drive, operate heavy machinery, or perform any other dangerous activities until you know how SUBLOCADE affects you. Buprenorphine can cause drowsiness and slow reaction times. SUBLOCADE can make you sleepy, dizzy, or lightheaded. This may happen more often in the first few days after your injection and when your dose is changed.
- You should not drink alcohol or take prescription or over-the-counter medicines that contain alcohol during treatment with SUBLOCADE, because this can lead to loss of consciousness or even death.
What are the possible side effects of SUBLOCADE?
SUBLOCADE can cause serious side effects, including:
- Trouble breathing. Taking other opioid medicines, benzodiazepines, alcohol, or other central nervous system depressants during treatment with SUBLOCADE can cause breathing problems that can lead to coma and death.
- Sleepiness, dizziness, and problems with coordination.
- Physical dependence.
- Liver problems. Call your healthcare provider right away if you notice any of these symptoms:
- your skin or the white part of your eyes turns yellow (jaundice)
- dark or “tea-colored” urine
- light colored stools (bowel movements)
- loss of appetite
- pain, aching, or tenderness on the right side of your stomach area
- nausea
- Your healthcare provider should do blood tests to check your liver before you start and during treatment with SUBLOCADE.
- Allergic reaction. You may have a rash, hives, swelling of your face, wheezing, low blood pressure, or loss of consciousness. Call your healthcare provider or get emergency help right away.
- Opioid withdrawal. Call your healthcare provider right away if you get any of these symptoms:
- shaking
- sweating more than normal
- feeling hot or cold more than normal
- runny nose
- watery eyes
- goose bumps
- diarrhea
- vomiting
- muscle aches
- Decrease in blood pressure. You may feel dizzy when you get up from sitting or lying down.
- The most common side effects of SUBLOCADE include:
- constipation
- headache
- nausea
- injection site itching
- vomiting
- increase in liver enzymes
- tiredness
- injection site pain
- SUBLOCADE may affect fertility in males and females. Talk to your healthcare provider if this is a concern for you.
These are not all the possible side effects of SUBLOCADE.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.General information about SUBLOCADE
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your doctor or pharmacist for information that is written for healthcare professionals.What are the ingredients in SUBLOCADE?
Active ingredient: buprenorphine
ATRIGEL® delivery system: biodegradable 50:50 poly(DL-lactide-co-glycolide) polymer and a biocompatible solvent, N-methyl-2-pyrrolidone (NMP).© 2022, Indivior UK Limited. All Rights Reserved.
SUBLOCADE® is a registered trademark of Indivior UK Limited.
Manufactured by Curia Global Inc. Albany, NY 12203
For more information, go to www.SUBLOCADE.com or call 1-877-782-6966. - Because of the serious risk of potential harm or death from self-injecting SUBLOCADE into a vein (intravenously), it is only available through a restricted program called the SUBLOCADE REMS Program.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SUBLOCADE
buprenorphine solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:12496-0100 Route of Administration SUBCUTANEOUS DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength buprenorphine (UNII: 40D3SCR4GZ) (buprenorphine - UNII:40D3SCR4GZ) buprenorphine 100 mg Inactive Ingredients Ingredient Name Strength POLY(DL-LACTIC-CO-GLYCOLIC ACID), (50:50; 15000 MW) (UNII: C329FPO0UC) Methylpyrrolidone (UNII: JR9CE63FPM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12496-0100-1 1 in 1 CARTON 02/26/2018 1 NDC:12496-0100-2 1 in 1 POUCH 1 NDC:12496-0100-5 1 in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209819 02/26/2018 SUBLOCADE
buprenorphine solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:12496-0300 Route of Administration SUBCUTANEOUS DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength buprenorphine (UNII: 40D3SCR4GZ) (buprenorphine - UNII:40D3SCR4GZ) buprenorphine 300 mg Inactive Ingredients Ingredient Name Strength POLY(DL-LACTIC-CO-GLYCOLIC ACID), (50:50; 15000 MW) (UNII: C329FPO0UC) Methylpyrrolidone (UNII: JR9CE63FPM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12496-0300-1 1 in 1 CARTON 02/26/2018 1 NDC:12496-0300-2 1 in 1 POUCH 1 NDC:12496-0300-5 1 in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209819 02/26/2018 Labeler - Indivior Inc. (797408549) Establishment Name Address ID/FEI Business Operations Curia Massachusetts, Inc. 556991748 MANUFACTURE(12496-0100, 12496-0300) Establishment Name Address ID/FEI Business Operations QUOTIENT SCIENCES (ALNWICK) LIMITED 228248590 ANALYSIS(12496-0100, 12496-0300) Establishment Name Address ID/FEI Business Operations Indivior UK Limited 220689622 API MANUFACTURE(12496-0100, 12496-0300) , ANALYSIS(12496-0100, 12496-0300) Establishment Name Address ID/FEI Business Operations Curia New Mexico, LLC. 078268840 ANALYSIS(12496-0100, 12496-0300) Establishment Name Address ID/FEI Business Operations Sharp Packaging Services, LLC 143696495 PACK(12496-0100, 12496-0300) Establishment Name Address ID/FEI Business Operations Sterling Pharma Solutions Limited 349157623 API MANUFACTURE(12496-0100, 12496-0300) , ANALYSIS(12496-0100, 12496-0300) Establishment Name Address ID/FEI Business Operations Wickham Micro Limited 228216353 ANALYSIS(12496-0100, 12496-0300) Establishment Name Address ID/FEI Business Operations Curia Global, Inc. 080046430 ANALYSIS(12496-0100, 12496-0300) Establishment Name Address ID/FEI Business Operations Curia New Mexico, LLC 078268825 ANALYSIS(12496-0100, 12496-0300) Establishment Name Address ID/FEI Business Operations Curia New Mexico, LLC 826977121 MANUFACTURE(12496-0100, 12496-0300) Establishment Name Address ID/FEI Business Operations Eurofins Lancaster Laboratories, LLC 069777290 ANALYSIS(12496-0100, 12496-0300) Establishment Name Address ID/FEI Business Operations Indivior Inc. 080660711 ANALYSIS(12496-0100, 12496-0300) Establishment Name Address ID/FEI Business Operations Nelson Laboratories, LLC 151663234 ANALYSIS(12496-0100, 12496-0300) Establishment Name Address ID/FEI Business Operations SGS North America Inc. 049859261 ANALYSIS(12496-0100, 12496-0300)