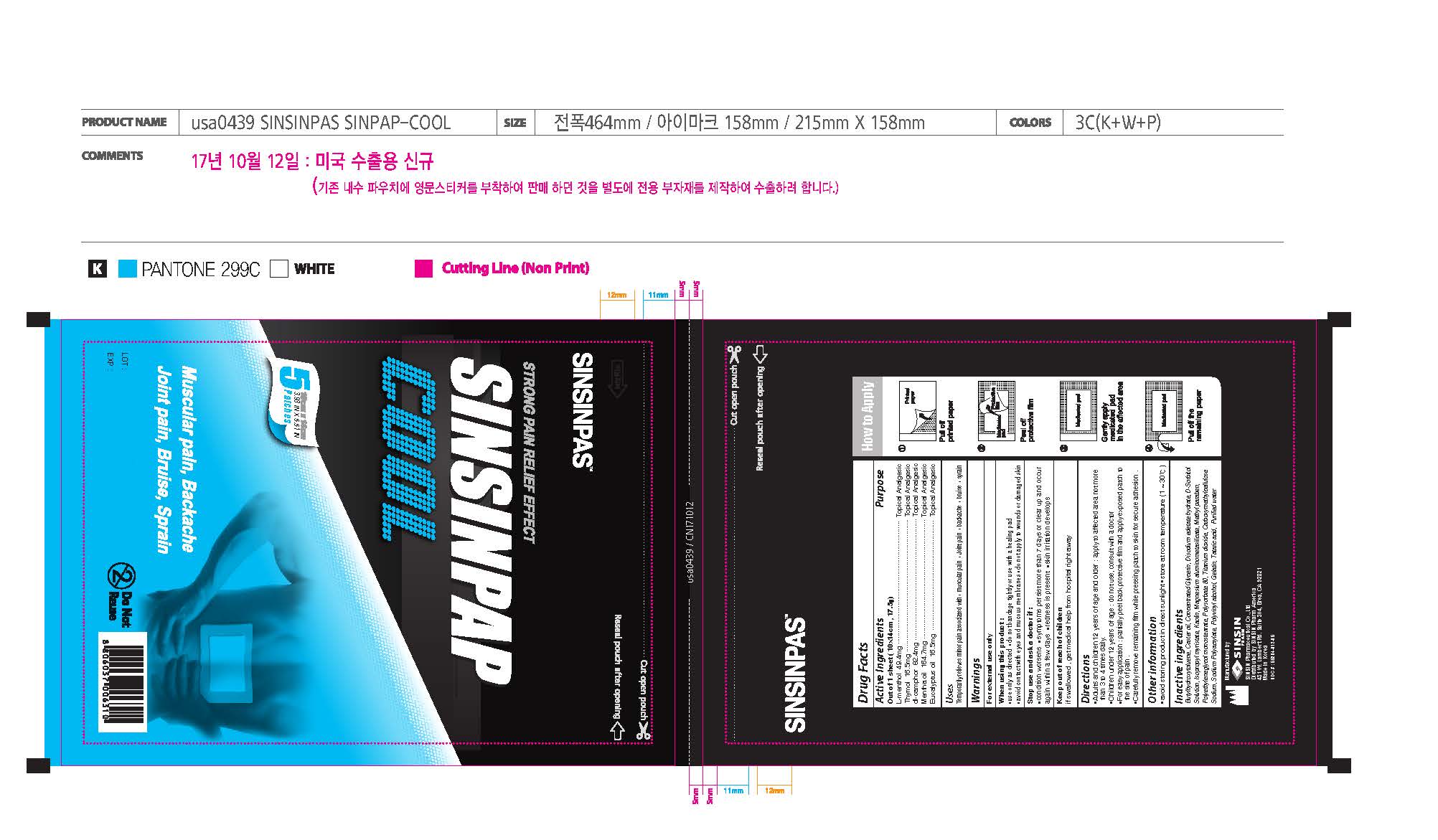
Label: SINSINPAP COOL- levomenthol, thymol, camphor, mentha, eucalyptus patch
- NDC Code(s): 55264-012-04, 55264-012-05
- Packager: Sinsin Pharmaceutical Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- When using this product:
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- Adults and children 12 years of age and over: apply to affected area not more than 3 to 4 times daily.
- Children under 12 years of age: do not use, consult a doctor
- For easy application: partially peel back protective film and apply exposed patch to the site of pain.
- Carefully remove remaining film while pressing patch to skin for secure adhesion.
- Storage condition
-
Inactive ingredients
BHT, Castor oil, Concentrated Glycerin, Disodium edetate hydrate, D-sorbitol, Isopropyl myristate, Kaolin, Magnesium aluminometasilicate, Methyl Paraben, PEG-4 stearate, Polysorbate 80, Titanium dioxide, Carboxymethylcellulose Sodium, Sodium Polyacrylate, Polyvinyl alchol, Gelatin, Tataric acid, Purified water
- Manufactured by:
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SINSINPAP COOL
levomenthol, thymol, camphor, mentha, eucalyptus patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55264-012 Route of Administration TOPICAL, CUTANEOUS, TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOMENTHOL (UNII: BZ1R15MTK7) (LEVOMENTHOL - UNII:BZ1R15MTK7) LEVOMENTHOL 49.4 mg THYMOL (UNII: 3J50XA376E) (THYMOL - UNII:3J50XA376E) THYMOL 16.5 mg CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 82.4 mg PEPPERMINT OIL (UNII: AV092KU4JH) (PEPPERMINT - UNII:V95R5KMY2B) PEPPERMINT OIL 164.7 mg EUCALYPTUS OIL (UNII: 2R04ONI662) (EUCALYPTUS OIL - UNII:2R04ONI662) EUCALYPTUS OIL 16.5 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CASTOR OIL (UNII: D5340Y2I9G) GLYCERIN (UNII: PDC6A3C0OX) EDETATE DISODIUM (UNII: 7FLD91C86K) SORBITOL (UNII: 506T60A25R) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) KAOLIN (UNII: 24H4NWX5CO) SILODRATE (UNII: 9T3UU8T0QK) METHYLPARABEN (UNII: A2I8C7HI9T) PEG-4 STEARATE (UNII: J33E8608YN) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TARTARIC ACID (UNII: W4888I119H) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55264-012-05 1 in 1 CARTON 05/30/2016 1 NDC:55264-012-04 5 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 05/30/2016 Labeler - Sinsin Pharmaceutical Co., Ltd. (823149161) Registrant - Sinsin Pharmaceutical Co., Ltd. (687867143) Establishment Name Address ID/FEI Business Operations Sinsin Pharmaceutical Co., Ltd. 687867143 manufacture(55264-012)