Label: BROMSITE 0.075%- bromfenac solution/ drops
- NDC Code(s): 49708-754-41
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BROMSITE® safely and effectively. See full prescribing information for BROMSITE.
BROMSITE (bromfenac ophthalmic solution) 0.075%, for topical ophthalmic use
Initial U.S. Approval: 1997INDICATIONS AND USAGE
BromSite is a nonsteroidal anti-inflammatory drug (NSAID) indicated for the treatment of postoperative inflammation and prevention of ocular pain in patients undergoing cataract surgery. (1) (1)
DOSAGE AND ADMINISTRATION
Instill one drop of BromSite to the affected eye twice daily (morning and evening) beginning 1 day prior to surgery, the day of surgery, and 14 days postsurgery. (2.1) (2)
DOSAGE FORMS AND STRENGTHS
Topical ophthalmic solution: bromfenac 0.075%. (3) (3)
CONTRAINDICATIONS
None (4) (4)
WARNINGS AND PRECAUTIONS
- •
- Slow or Delayed Healing (5.1)
- •
- Potential for Cross-Sensitivity (5.2)
- •
- Increased Bleeding Time of Ocular Tissue (5.3)
- •
- Keratitis and Corneal Effects (5.4)
- •
- Contact Lens Wear (5.5)
ADVERSE REACTIONS
The most commonly reported adverse reactions in 1-8% of patients were: anterior chamber inflammation, headache, vitreous floaters, iritis, eye pain and ocular hypertension. (6.1) (6)
To report SUSPECTED ADVERSE REACTIONS, contact Sun Pharmaceutical Industries, Inc. at 1-800-406-7984 (toll free), or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Use with Other Topical Ophthalmic Medications
3 DOSAGE FORM AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Slow or Delayed Healing
5.2 Potential for Cross-Sensitivity
5.3 Increased Bleeding Time of Ocular Tissue
5.4 Keratitis and Corneal Reactions
5.5 Contact Lens Wear
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
14 CLINICAL STUDIES
14.1 Ocular Inflammation and Pain
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
One drop of BromSite should be applied to the affected eye twice daily (morning and evening) 1 day prior to surgery, the day of surgery, and 14 days postsurgery.
2.2 Use with Other Topical Ophthalmic Medications
BromSite should be administered at least 5 minutes after instillation of other topical medications. BromSite may be administered in conjunction with other topical ophthalmic medications such as alpha-agonists, beta-blockers, carbonic anhydrase inhibitors, cycloplegics, and mydriatics.
- 3 DOSAGE FORM AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Slow or Delayed Healing
All topical nonsteroidal anti-inflammatory drugs (NSAIDs), including BromSite (bromfenac ophthalmic solution) 0.075%, may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems.
5.2 Potential for Cross-Sensitivity
There is the potential for cross-sensitivity to acetylsalicylic acid, phenylacetic acid derivatives, and other NSAIDs, including BromSite (bromfenac ophthalmic solution) 0.075%. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
5.3 Increased Bleeding Time of Ocular Tissue
With some NSAIDs, including BromSite (bromfenac ophthalmic solution) 0.075%, there exists the potential for increased bleeding time due to interference with platelet aggregation. There have been reports that ocularly applied NSAIDs may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery.
It is recommended that BromSite be used with caution in patients with known bleeding tendencies or who are receiving other medications which may prolong bleeding time.
5.4 Keratitis and Corneal Reactions
Use of topical NSAIDs may result in keratitis. In some susceptible patients, continued use of topical NSAIDs may result in epithelial breakdown, corneal thinning, corneal erosion, corneal ulceration or corneal perforation. These events may be sight threatening. Patients with evidence of corneal epithelial breakdown should immediately discontinue use of topical NSAIDs, including BromSite (bromfenac ophthalmic solution) 0.075%, and should be closely monitored for corneal health.
Postmarketing experience with topical NSAIDs suggests that patients with complicated ocular surgeries, corneal denervation, corneal epithelial defects, diabetes mellitus, ocular surface diseases (e.g., dry eye syndrome), rheumatoid arthritis, or repeat ocular surgeries within a short period of time may be at increased risk for corneal adverse events which may become sight threatening. Topical NSAIDs should be used with caution in these patients.
Postmarketing experience with topical NSAIDs also suggests that use more than 24 hours prior to surgery or use beyond 14 days postsurgery may increase patient risk for the occurrence and severity of corneal adverse events.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- •
- Slow or Delayed Healing [see Warnings and Precautions (5.1)]
- •
- Potential for Cross-Sensitivity [see Warnings and Precautions (5.2)]
- •
- Increased Bleeding Time of Ocular Tissue [see Warnings and Precautions (5.3)]
- •
- Keratitis and Corneal Reactions[see Warnings and Precautions (5.4)]
- •
- Contact Lens Wear [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The most commonly reported adverse reactions in 1 to 8% of patients were: anterior chamber inflammation, headache, vitreous floaters, iritis, eye pain and ocular hypertension. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies in pregnant women to inform any drug associated risks. Treatment of pregnant rats and rabbits with oral bromfenac did not produce teratogenic effects at clinically relevant doses.
Clinical Considerations
Because of the known effects of prostaglandin biosynthesis-inhibiting drugs on the fetal cardiovascular system (closure of ductus arteriosus), the use of BromSite during late pregnancy should be avoided.Data
Animal Data
Treatment of rats with bromfenac at oral doses up to 0.9 mg/kg/day (195 times a unilateral daily human ophthalmic dose on a mg/m2 basis, assuming 100% absorbed) and rabbits at oral doses up to 7.5 mg/kg/day (3243 times a unilateral daily dose on a mg/m2 basis) produced no structural teratogenicity in reproduction studies. However, embryo-fetal lethality, neonatal mortality and reduced postnatal growth were produced in rats at 0.9 mg/kg/day, and embryo-fetal lethality was produced in rabbits at 7.5 mg/kg/day. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.8.2 Lactation
There are no data on the presence of bromfenac in human milk, the effects on the breastfed infant, or the effects on milk production; however, systemic exposure to bromfenac from ocular administration is low [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for bromfenac and any potential adverse effects on the breast-fed child from bromfenac or from the underlying maternal condition.
-
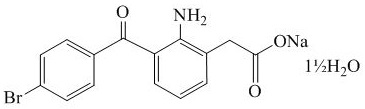
11 DESCRIPTION
BromSite (bromfenac ophthalmic solution) 0.075% is a sterile aqueous, topical NSAID, formulated in DuraSite® for ophthalmic use. The USAN name for bromfenac sodium sesquihydrate is bromfenac sodium. Bromfenac sodium is designated chemically as sodium [2-amino-3-(4-bromobenzoyl) phenyl] acetate sesquihydrate, with an empirical formula of C15H11BrNNaO3• 1½H2O. The structural formula for bromfenac sodium sesquihydrate is:
Bromfenac sodium is a bright orange to yellow powder. The molecular weight of bromfenac sodium sesquihydrate is 383.17. BromSite is a greenish-yellow to dark yellow viscous liquid with an osmolality of approximately 290 mOsmol/kg.
Active: Each mL contains bromfenac sodium sesquihydrate 0.87 mg, which is equivalent to bromfenac free acid 0.76 mg.
Preservative: benzalkonium chloride 0.005%
Inactives: boric acid, sodium borate, citric acid anhydrous, sodium citrate dihydrate, poloxamer 407, polycarbophil, sodium chloride, edetate disodium dihydrate, sodium hydroxide (to adjust pH to 8.3), and water for injection (USP). -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Bromfenac is a nonsteroidal anti-inflammatory drug (NSAID) that has anti-inflammatory activity. The mechanism of its action is thought to be due to its ability to block prostaglandin synthesis by inhibiting cyclooxygenase 1 and 2. Prostaglandins have been shown in many animal models to be mediators of certain kinds of intraocular inflammation. In studies performed in animal eyes, prostaglandins have been shown to produce disruption of the blood-aqueous humor barrier, vasodilation, increased vascular permeability, leukocytosis, and increased intraocular pressure.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
Long-term carcinogenicity studies in rats and mice given oral doses of bromfenac up to 0.6 mg/kg/day (129 times a unilateral daily dose assuming 100% absorbed, on a mg/m2 basis) and 5 mg/kg/day (540 times a unilateral daily dose on a mg/m2 basis), respectively revealed no significant increases in tumor incidence.
Bromfenac did not show mutagenic potential in various mutagenicity studies, including the bacterial reverse mutation, chromosomal aberration, and micronucleus tests.
Bromfenac did not impair fertility when administered orally to male and female rats at doses up to 0.9 mg/kg/day and 0.3 mg/kg/day, respectively (195 and 65 times a unilateral daily dose, respectively, on a mg/m2 basis). -
14 CLINICAL STUDIES
14.1 Ocular Inflammation and Pain
Clinical efficacy was evaluated in 2 multi-centered, randomized, double-masked, parallel group, placebo-controlled US trials in which subjects requiring cataract surgery were assigned to receive BromSite or vehicle. Patients undergoing cataract surgery self-administered BromSite or vehicle twice daily, beginning 1 day prior to surgery, continuing the day of surgery and for 14 days after surgery. Clearance of ocular inflammation was assessed on Days 1, 8, 15, and 29 using slit lamp biomicroscopy. The primary efficacy endpoint was the proportion of subjects with anterior chamber cell (ACC) grade 0 at Day 15. The secondary efficacy endpoint was the proportion of subjects who were pain free after cataract surgery as assessed using a Visual Analog Scale.
Proportion of Subjects with Cleared Ocular Inflammation, ACC Grade 0
Visit
BromSite
Vehicle
Treatment Difference (95% CI)
Study 1
Day 8
54/168 (32.1%)
7/85 (8.2%)
23.9% (14.7%, 33.1%)
Day 15
96/168 (57.1%)
16/85 (18.8%)
38.3% (27.1%, 49.5%)
Study 2
Day 8
40/168 (23.8%)
8/85 (9.4%)
1 4.4% (5.5%, 23.3%)
Day 15
64/168 (38.1%)
19/85 (22.4%)
1 5.7% (4.2%, 27.3%)
Proportion of Subjects who were Pain Free
Study 1
Day 1
129/168 (76.8%)
41/85 (48.2%)
28.6% (16.2%, 40.9%)
Study 2
Day 1
138/168 (82.1%)
53/85 (62.4%)
19.8% (8.0%, 31.6%)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
BromSite (bromfenac ophthalmic solution) 0.075% is supplied in white opaque low density polyethylene (LDPE) plastic bottles and translucent dropper tips, and gray high density polyethylene (HDPE) eyedropper caps. A white tamper evident overcap is provided. Each bottle is provided in a sealed foil laminated pouch.
5 mL in a 7.5 mL bottle
(NDC No. 49708-754-41)
STORAGE
Store at 15°C to 25°C (59°F to 77°F). After opening, BromSite can be used until the expiration date on the bottle. Discard after treatment completion. -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Slow or Delayed Healing
Advise patients of the possibility that slow or delayed healing may occur while using NSAIDs.
Concomitant Topical Ocular Therapy
If more than one topical ophthalmic medication is being used, advise patients to administer BromSite at least 5 minutes after instillation of other topical medications.
Concomitant Use of Contact Lenses
Advise patients not to wear contact lenses during administration of BromSite. The preservative in this product, benzalkonium chloride, may be absorbed by soft contact lenses.Sterility of Dropper Tip/Product Use
Advise patients to replace the bottle cap after use and do not touch the dropper tip to any surface as this may contaminate the contents.
Advise patients to thoroughly wash hands prior to using BromSite.Distributed by:
Sun Pharmaceutical Industries, Inc.
Cranbury, NJ 08512US Patent No. 8,778,999
BromSite and DuraSite are registered trademarks of Sun Pharmaceutical Industries Limited
© 2022 Sun Pharmaceutical Industries, Inc. All rights reserved.
Rev: MM/YYYY
VISX001-642R02
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
BromSite®[BRŏM- sahyt]
(bromfenac ophthalmic solution) 0.075%Read this Instructions for Use before you start using BromSite and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or treatment.
Information about BromSite:
• Do not let the BromSite applicator tip touch your eye, fingers, or any other surfaces.
• If you are using BromSite with other eye (ophthalmic) medicines, you should wait at least 5 minutes after using the other medicine to give your BromSite dose.
• You should not wear contact lenses while using BromSite.
• Put the gray cap back on the BromSite after each use.Before you use BromSite for the first time:
• Tear open the foil pouch using the perforated notch and remove the BromSite bottle. Throw away the foil pouch.
• Remove the white cap by turning it in the clockwise direction (See FIGURE A). Throw away the white cap.
• Hold the bottle upright. Remove the gray cap by turning it in the counterclockwise direction (See FIGURE B).
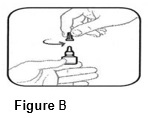
• Replace the gray cap on the bottle and close tightly.
Follow Steps 1 to 5 each time you use BromSite.
Step 1. Wash your hands well.
Step 2. Turn the closed bottle upside down (See FIGURE C).
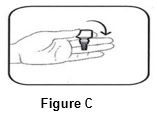
Step 3. Flick bottle firmly 1 time before each use to move the medicine into the tip of the bottle (See FIGURE D).
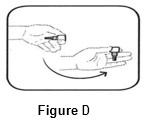
Step 4. Keep the bottle upside down and remove the gray cap by turning it in the clockwise direction (See FIGURE E).
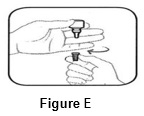
Step 5. Tilt your head back. Gently squeeze the bottle to place 1 drop into the affected eye (See FIGURE F). Replace the gray cap on the bottle and close tightly.
How do I store BromSite?
• Store at 15°C to 25°C (59°F to 77°F). After opening, BromSite can be used until the expiration date on the bottle.
• Throw away the BromSite bottle after your treatment is finished.This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
Sun Pharmaceutical Industries, Inc.
Cranbury, NJ 08512
US Patent No. 8,778,999BromSite and DuraSite are registered trademarks of Sun Pharmaceutical Industries Limited
© 2022 Sun Pharmaceutical Industries, Inc. All rights reservedRev.: 03/2023
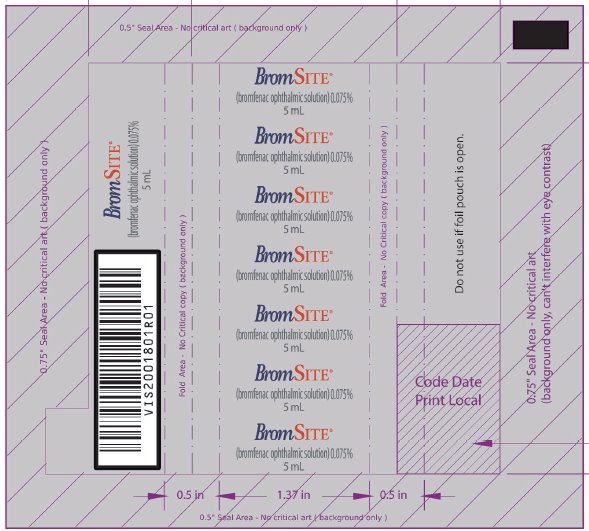
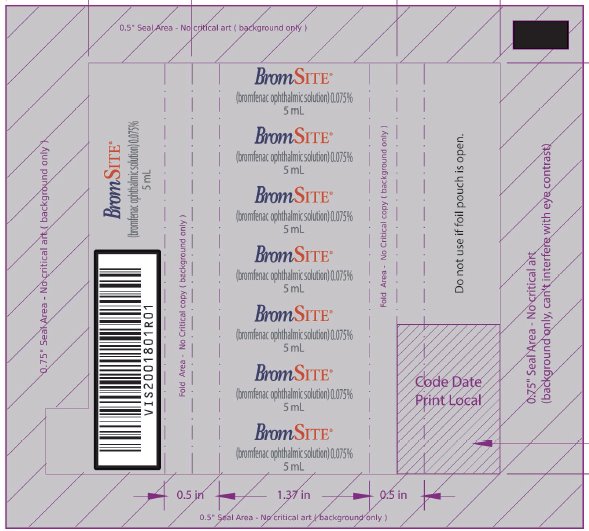
VISX001650R01 - PRINCIPAL DISPLAY PANEL - NDC: 49708-754-41 - 5 ML FOIL WRAPPER
- PRINCIPAL DISPLAY PANEL - NDC: 49708-754-41 - 5 ML BOTTLE LABEL
- PRINCIPAL DISPLAY PANEL - NDC: 49708-754-41 - 5 ML CARTON LABEL
-
INGREDIENTS AND APPEARANCE
BROMSITE 0.075%
bromfenac solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49708-754 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BROMFENAC SODIUM (UNII: 8ECV571Y37) (BROMFENAC - UNII:864P0921DW) BROMFENAC 0.76 mg in 1 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM BORATE (UNII: 91MBZ8H3QO) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) POLOXAMER 407 (UNII: TUF2IVW3M2) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) 0.05 mg in 1 mL POLYCARBOPHIL (UNII: W25LM17A4W) SODIUM CHLORIDE (UNII: 451W47IQ8X) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Product Characteristics Color GREEN, YELLOW Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49708-754-41 1 in 1 CARTON 05/01/2016 1 1 in 1 POUCH 1 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206911 05/01/2016 Labeler - Sun Pharmaceutical Industries, Inc. (146974886) Establishment Name Address ID/FEI Business Operations Woodstock Sterile Solutions, Inc. 117895702 ANALYSIS(49708-754) , LABEL(49708-754) , MANUFACTURE(49708-754) , PACK(49708-754)