Label: CINQAIR- reslizumab injection, solution, concentrate
- NDC Code(s): 59310-610-31, 59310-610-33
- Packager: Teva Respiratory, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 29, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CINQAIR safely and effectively. See full prescribing information for CINQAIR.
CINQAIR® (reslizumab) injection, for intravenous use
Initial U.S. Approval: 2016
WARNING: ANAPHYLAXIS
See full prescribing information for complete boxed warning.
- Anaphylaxis occurred with CINQAIR infusion in 0.3% of patients in placebo-controlled studies (5.1)
- Patients should be observed for an appropriate period of time after CINQAIR infusion; healthcare professionals should be prepared to manage anaphylaxis that can be life-threatening (5.1)
- Discontinue CINQAIR immediately if the patient experiences anaphylaxis (5.1)
INDICATIONS AND USAGE
CINQAIR is an interleukin-5 antagonist monoclonal antibody (IgG4 kappa) indicated for add-on maintenance treatment of patients with severe asthma aged 18 years and older, and with an eosinophilic phenotype (1).
Limitations of Use: CINQAIR is not indicated for:
DOSAGE AND ADMINISTRATION
- CINQAIR is for intravenous infusion only. Do not administer as an intravenous push or bolus (2.1)
- CINQAIR should be administered by a healthcare professional prepared to manage anaphylaxis (2.2)
- Recommended dosage regimen is 3 mg/kg once every 4 weeks by intravenous infusion over 20-50 minutes (2.1)
DOSAGE FORMS AND STRENGTHS
Injection: 100 mg/10 mL (10 mg/mL) solution in single-use vials (3)
CONTRAINDICATIONS
Known hypersensitivity to reslizumab or any of its excipients (4)
WARNINGS AND PRECAUTIONS
- Malignancy: Malignancies were observed in clinical studies. (5.3)
- Reduction in Corticosteroid Dosage: Do not discontinue systemic or inhaled corticosteroids abruptly upon initiation of therapy with CINQAIR. Decrease corticosteroids gradually, if appropriate. (5.4)
- Parasitic (Helminth) Infection: Treat patients with pre-existing helminth infections before therapy with CINQAIR. If patients become infected while receiving CINQAIR and do not respond to anti-helminth treatment, discontinue CINQAIR until the parasitic infection resolves. (5.5)
ADVERSE REACTIONS
The most common adverse reaction (incidence greater than or equal to 2%) includes oropharyngeal pain. (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals at 1-888-483-8279 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: ANAPHYLAXIS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing
2.2 Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylaxis
5.2 Acute Asthma Symptoms or Deteriorating Disease
5.3 Malignancy
5.4 Reduction of Corticosteroid Dosage
5.5 Parasitic (Helminth) Infection
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: ANAPHYLAXIS
Anaphylaxis has been observed with CINQAIR infusion in 0.3% of patients in placebo-controlled clinical studies. Anaphylaxis was reported as early as the second dose of CINQAIR [see Warnings and Precautions (5.1), Adverse Reactions (6)].
Anaphylaxis can be life-threatening. Patients should be observed for an appropriate period of time after CINQAIR administration by a healthcare professional prepared to manage anaphylaxis. Discontinue CINQAIR immediately if the patient experiences signs or symptoms of anaphylaxis [see Dosage and Administration (2.2), Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
CINQAIR® is indicated for the add-on maintenance treatment of patients with severe asthma aged 18 years and older with an eosinophilic phenotype [see Clinical Studies (14)].
Limitation of Use:
- CINQAIR is not indicated for treatment of other eosinophilic conditions.
- CINQAIR is not indicated for the relief of acute bronchospasm or status asthmaticus [see Warnings and Precautions (5.2)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing
CINQAIR is for intravenous infusion only. Do not administer as an intravenous push or bolus.
The recommended dosage regimen is 3 mg/kg once every 4 weeks administered by intravenous infusion over 20-50 minutes [see Dosage and Administration (2.2)].
Discontinue the infusion immediately if the patient experiences a severe systemic reaction, including anaphylaxis [see Contraindications (4), Warnings and Precautions (5.1)].
2.2 Preparation and Administration Instructions
CINQAIR is provided as a solution in a single-use vial for intravenous infusion only and should be prepared by a healthcare professional using aseptic technique as follows:
Preparation of intravenous infusion
- Remove CINQAIR from the refrigerator. To minimize foaming, do not shake CINQAIR.
- Inspect visually for particulate matter and discoloration prior to administration. CINQAIR solution is clear to slightly hazy/opalescent, colorless to slightly yellow liquid. Since CINQAIR is a protein, proteinaceous particles may be present in the solution that appear as translucent to white, amorphous particulates. Do not administer if discolored or if other foreign particulate matter is present.
- Withdraw the proper volume of CINQAIR from the vial(s), based on the recommended weight-based dosage. Discard any unused portion.
- Dispense syringe contents slowly into an infusion bag containing 50 mL of 0.9% Sodium Chloride Injection, USP to minimize foaming of CINQAIR (CINQAIR is compatible with polyvinylchloride (PVC) or polyolefin infusion bags). Gently invert the bag to mix the solution. Do not shake. Do not mix or dilute with other drugs.
- Administer immediately after preparation. If not used immediately, store diluted solutions of CINQAIR in the refrigerator at 2°C to 8°C (36°F to 46°F) or at room temperature up to 25ºC (77ºF), protected from light, for up to 16 hours. The time between preparation of CINQAIR and administration should not exceed 16 hours.
Administration instructions
- CINQAIR should be administered by a healthcare professional prepared to manage anaphylaxis [see Warnings and Precautions (5.1)].
- If refrigerated prior to administration, allow the diluted CINQAIR solution to reach room temperature.
- Use an infusion set with an in-line, low protein-binding filter (pore size of 0.2 micron). CINQAIR is compatible with polyethersulfone (PES), polyvinylidene fluoride (PVDF), nylon, and cellulose acetate in-line infusion filters.
- Infuse the diluted solution of CINQAIR intravenously, over a 20 to 50 minute period. Infusion time may vary depending on the total volume to be infused as based upon patient weight.
- Do not infuse CINQAIR concomitantly in the same intravenous line with other agents. No physical or biochemical compatibility studies have been conducted to evaluate the co-administration of CINQAIR with other agents.
- Observe the patient over the infusion and for an appropriate period of time following infusion.
- Upon completion of the infusion, flush the intravenous administration set with 0.9% Sodium Chloride Injection, USP to ensure that all CINQAIR has been administered.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
CINQAIR is contraindicated in patients who have known hypersensitivity to reslizumab or any of its excipients [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Anaphylaxis
Anaphylaxis to CINQAIR was reported in 0.3% of asthma patients in placebo-controlled clinical studies [see Adverse Reactions (6.1)]. These events were observed during or within 20 minutes after completion of the CINQAIR infusion and reported as early as the second dose of CINQAIR. Manifestations included dyspnea, decreased oxygen saturation, wheezing, vomiting, and skin and mucosal involvement, including urticaria. In all 3 cases, CINQAIR was discontinued.
Anaphylaxis can be life-threatening. CINQAIR should be administered by a healthcare professional prepared to manage anaphylaxis. Patients should be observed for an appropriate period of time after CINQAIR administration. If severe systemic reactions, including anaphylaxis, occur, stop administration of CINQAIR immediately and provide appropriate medical treatment. Prior to CINQAIR administration, inform patients of the signs and symptoms of anaphylaxis and instruct them to seek immediate medical care if symptoms occur. Discontinue CINQAIR use permanently if the patient experiences signs or symptoms of anaphylaxis [see Contraindications (4)].
5.2 Acute Asthma Symptoms or Deteriorating Disease
CINQAIR should not be used to treat acute asthma symptoms or acute exacerbations. Do not use CINQAIR to treat acute bronchospasm or status asthmaticus. Patients should seek medical advice if their asthma remains uncontrolled or worsens after initiation of treatment with CINQAIR.
5.3 Malignancy
In placebo-controlled clinical studies, 6/1028 (0.6%) patients receiving 3 mg/kg CINQAIR had at least 1 malignant neoplasm reported compared to 2/730 (0.3%) patients in the placebo group. The observed malignancies in CINQAIR-treated patients were diverse in nature and without clustering of any particular tissue type. The majority of malignancies were diagnosed within less than six months of exposure to CINQAIR.
5.4 Reduction of Corticosteroid Dosage
No clinical studies have been conducted to assess reduction of maintenance corticosteroid dosages following administration of CINQAIR. Do not discontinue systemic or inhaled corticosteroids abruptly upon initiation of therapy with CINQAIR. Reductions in corticosteroid dose, if appropriate, should be gradual and performed under the supervision of a physician. Reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy.
5.5 Parasitic (Helminth) Infection
Eosinophils may be involved in the immunological response to some helminth infections. Patients with known parasitic infections were excluded from participation in clinical studies. It is unknown if CINQAIR will influence the immune response against parasitic infections. Treat patients with pre-existing helminth infections before initiating CINQAIR. If patients become infected while receiving treatment with CINQAIR and do not respond to anti-helminth treatment, discontinue treatment with CINQAIR until infection resolves.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in other sections of the labeling:
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Overall, 2195 subjects received at least 1 dose of CINQAIR. The data described below reflect exposure to CINQAIR in 1611 patients with asthma, including 1120 exposed for up to 16 weeks, 1006 exposed for 6 months, 759 exposed for 1 year, and 249 exposed for longer than 2 years. The above referenced safety exposure for CINQAIR is derived from placebo-controlled studies ranging from 15 to 52 weeks in duration (CINQAIR 0.3 mg/kg and 3 mg/kg [n=1131] and placebo [n=730]) and 480 new CINQAIR 3 mg/kg exposures (previously on placebo) from a single open-label extension study (n=1051). While a lower dose of CINQAIR 0.3 mg/kg (n=103) was included in a clinical trial, 3 mg/kg is the only recommended dose [see Dosage and Administration (2.1)]. Of the 1611 patients, 1596 received the 3 mg/kg dose, 1028 of which were in the placebo-controlled studies. In the placebo-controlled asthma studies, the population studied was 12 to 76 years of age, 62% female, and 73% white. While subjects aged 12 to 17 years were included in these trials, CINQAIR is not approved for use in this age group [see Use in Specific Populations (8.4)].
Serious adverse reactions that occurred in placebo-controlled studies in more than 1 subject and in a greater percentage of subjects treated with CINQAIR (n=1131) than placebo (n=730) included anaphylaxis (3 subjects vs. 0 subjects, respectively). The 3 subjects who experienced anaphylaxis were discontinued from the clinical studies [see Warnings and Precautions (5.1)]. Malignancy also occurred more commonly in patients treated with CINQAIR than placebo (0.6% and 0.3%, respectively) [see Warnings and Precautions (5.3)].
Adverse reactions that occurred at greater than or equal to 2% incidence and more commonly than in the placebo group included 1 event: oropharyngeal pain (2.6% vs. 2.2%).
CPK elevations and muscle-related adverse reactions
Elevated baseline creatine phosphokinase (CPK) was more frequent in patients randomized to CINQAIR (14%) versus placebo (9%). Transient CPK elevations in patients with normal baseline CPK values were observed more frequently with CINQAIR (20%) versus placebo (18%) during routine laboratory assessments. CPK elevations >10 x ULN, regardless of baseline CPK value, were 0.8% in the CINQAIR group compared to 0.4% in the placebo group. CPK elevations >10 x ULN were asymptomatic and did not lead to treatment discontinuation.
Myalgia was reported in 1% (10/1028) of patients in the CINQAIR 3 mg/kg group compared to 0.5% (4/730) of patients in the placebo group. On the day of infusion, musculoskeletal adverse reactions were reported in 2.2% and 1.5% of patients treated with CINQAIR 3 mg/kg and placebo, respectively. These reactions included (but were not limited to) musculoskeletal chest pain, neck pain, muscle spasms, extremity pain, muscle fatigue, and musculoskeletal pain.
6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. In placebo-controlled studies, a treatment-emergent anti-reslizumab antibody response developed in 53/983 (5.4%) of CINQAIR-treated patients (3 mg/kg). In the long-term, open-label study, treatment-emergent anti-reslizumab antibodies were detected in 49/1014 (4.8%) of CINQAIR-treated (3 mg/kg) asthma patients over 36 months. The antibody responses were of low titer and often transient. Neutralizing antibodies were not evaluated. There was no detectable impact of the antibodies on the clinical pharmacokinetics, pharmacodynamics, clinical efficacy, and safety of CINQAIR [see Clinical Pharmacology (12.2)]. Product-specific IgE antibodies were not detected in patients who reported anaphylactic reactions.
The data reflect the percentage of patients whose test results were positive for antibodies to reslizumab in specific assays. The observed incidence of antibody response is highly dependent on several factors, including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medication, and underlying disease. For these reasons, comparison of the incidence of antibodies to reslizumab with the incidence of antibodies to other products may be misleading.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The data on pregnancy exposure from the clinical trials are insufficient to inform on drug-associated risk. Monoclonal antibodies, such as reslizumab, are transported across the placenta in a linear fashion as pregnancy progresses; therefore, potential effects on a fetus are likely to be greater during the second and third trimester of pregnancy. Reslizumab has a long half-life [see Clinical Pharmacology (12.3)]. This should be taken into consideration.
In animal reproduction studies, there was no evidence of embryo-fetal adverse developmental effects with intravenous administration of reslizumab during organogenesis to pregnant mice and rabbits at doses that produced exposures up to approximately 6 times the exposure at the maximum recommended human dose (MRHD) in mice and approximately 17 times the exposure at the MRHD in rabbits [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population(s) are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo-Fetal Risk
In women with poorly or moderately controlled asthma, evidence demonstrates that there is an increased risk of preeclampsia in the mother and prematurity, low birth weight, and small for gestational age in the neonate. The level of asthma control should be closely monitored in pregnant women and treatment adjusted as necessary to maintain optimal control.
Data
Animal Data
In 2 separate embryo-fetal development studies, pregnant mice and rabbits received a single reslizumab dose during the period of organogenesis at 2, 10, and 50 mg/kg (0.4, 1.5, and 6 times the exposures achieved at the MRHD in mice on an AUC basis and 0.67, 3.3, and 17 times the exposures achieved at the MRHD in rabbits on a mg/kg basis). Reslizumab was not teratogenic in mice or rabbits. Embryo-fetal development of interleukin-5 (IL-5) deficient mice has been reported to be generally unaffected relative to wild-type mice.
In a prenatal and postnatal development study, pregnant CD-1 mice received reslizumab during organogenesis on gestation days 6 and 18 and on postnatal day 14 at 10 or 50 mg/kg (1.5 and 6 times the exposures achieved at the MRHD on an AUC basis). Reslizumab did not have any effects on fetal development up to approximately 4 months after birth. Reslizumab crossed the placenta of pregnant mice. Serum concentrations in pups were approximately 6 to 8% of those in the dams (parental female mice) on postnatal day 14.
8.2 Lactation
Risk Summary
It is not known whether reslizumab is present in human milk, and the effects of reslizumab on the breast fed infant and on milk production are not known. However, human IgG is known to be present in human milk. Reslizumab was present in the milk of lactating mice following dosing during pregnancy [see Data]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CINQAIR and any potential adverse effects on the breast-fed child from CINQAIR or the underlying maternal condition.
Data
Reslizumab was excreted in milk of lactating CD-1 mice that received reslizumab at 10 or 50 mg/kg (1.5 and 6 times the exposures achieved at the MRHD on an AUC basis) during pregnancy on gestation days 6 and 18 and on postnatal day 14. Levels of reslizumab in milk were approximately 5-7% of maternal serum concentrations.
8.4 Pediatric Use
CINQAIR is not indicated for use in pediatric patients less than 18 years of age. The safety and effectiveness in pediatric patients (aged 17 years and younger) have not been established.
CINQAIR was evaluated in 39 patients aged 12 to less than 18 years with asthma in two 52-week exacerbation studies and one 16-week lung function study. In the exacerbation studies, patients were required to have at least 1 asthma exacerbation requiring systemic corticosteroid use in the year prior to study entry. In these studies, the asthma exacerbation rate was higher in adolescent patients treated with CINQAIR than placebo (CINQAIR n=14, rate 2.86, 95% CI [1.02 to 8.09] and placebo n=11, rate 1.37, 95% CI [0.57 to 3.28]: rate ratio 2.09, 95% CI [0.82 to 5.36]).
8.5 Geriatric Use
CINQAIR was evaluated in 122 patients aged 65 years and older with asthma in two 52-week exacerbation studies and two 16-week lung function studies. No overall differences in safety or effectiveness were observed between these patients and younger patients. Based on available data, no adjustment of the dosage of CINQAIR in geriatric patients is necessary.
-
10 OVERDOSAGE
Single doses of up to 732 mg have been administered intravenously to subjects in clinical trials without evidence of dose-related toxicities.
There is no specific treatment for an overdose with CINQAIR. If overdose occurs, the patient should be treated supportively with appropriate monitoring as necessary.
-
11 DESCRIPTION
CINQAIR (reslizumab) is a humanized interleukin-5 antagonist monoclonal antibody (IgG4κ). Reslizumab is produced by recombinant DNA technology in murine myeloma non-secreting 0 (NS0) cells. Reslizumab has a molecular weight of approximately 147 kDa.
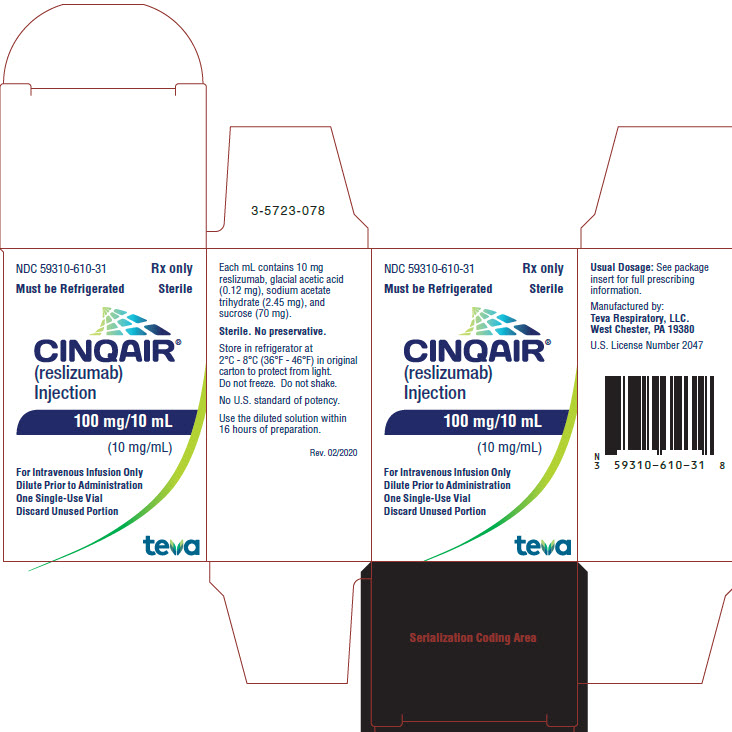
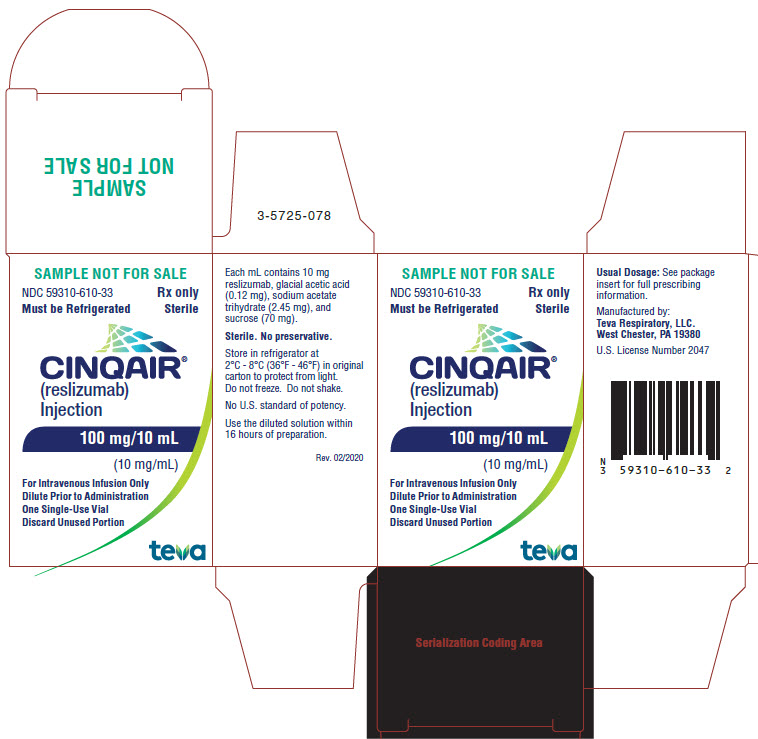
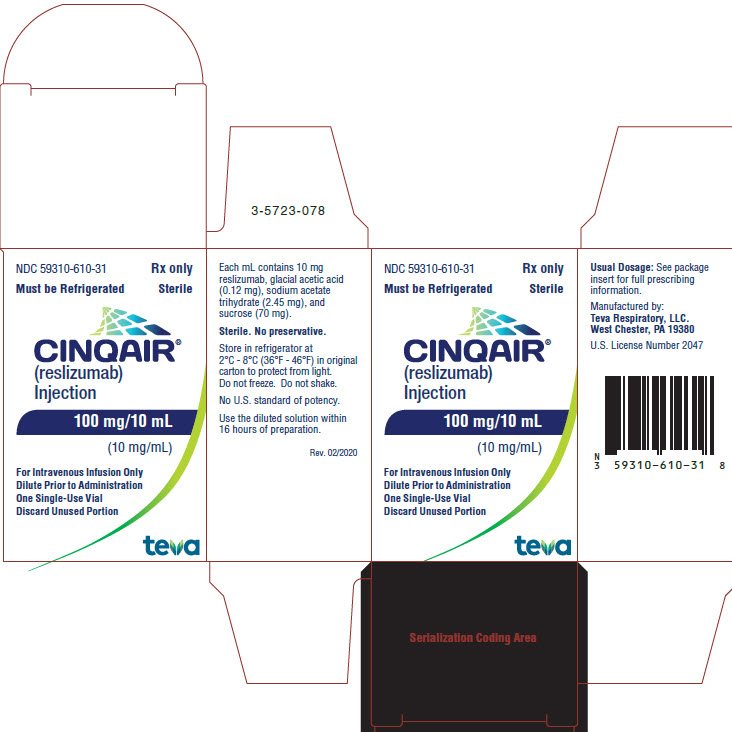
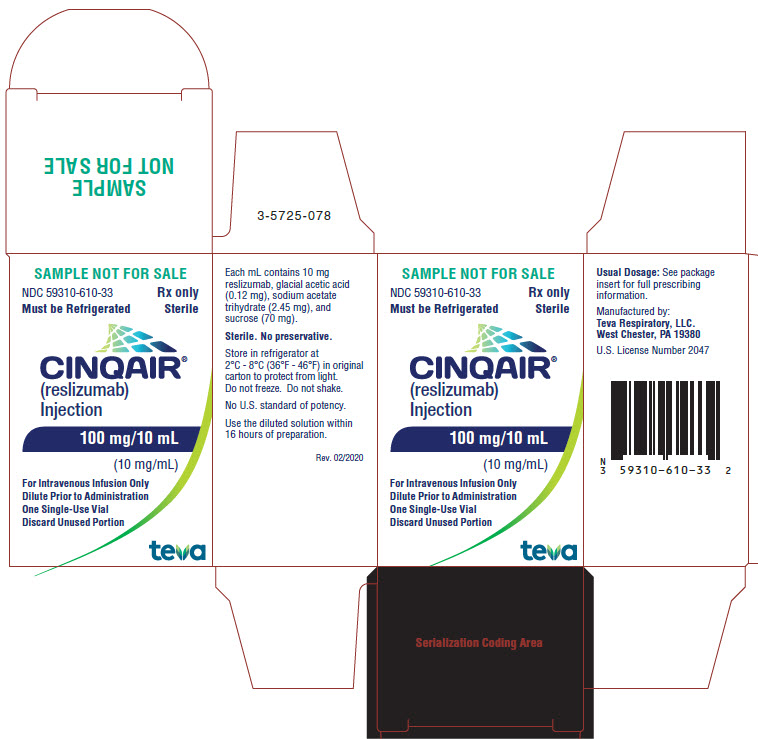
CINQAIR is a sterile, preservative-free, clear to slightly hazy/opalescent, colorless to slightly yellow solution (injection) for intravenous infusion. Since CINQAIR is a protein, proteinaceous particles may be present in the solution that appear as translucent to white, amorphous particulates. Each single-use vial contains 100 mg reslizumab in 10 mL. Each mL contains 10 mg of reslizumab, glacial acetic acid (0.12 mg), sodium acetate trihydrate (2.45 mg), and sucrose (70 mg), with a pH of 5.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Reslizumab is an interleukin-5 antagonist (IgG4, kappa). IL-5 is the major cytokine responsible for the growth and differentiation, recruitment, activation, and survival of eosinophils. Reslizumab binds to IL-5 with a dissociation constant of 81 pM, inhibiting the bioactivity of IL-5 by blocking its binding to the alpha chain of the IL-5 receptor complex expressed on the eosinophil surface. Inflammation is an important component in the pathogenesis of asthma. Multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, cytokines) are involved in inflammation. Reslizumab, by inhibiting IL-5 signaling, reduces the production and survival of eosinophils; however, the mechanism of reslizumab action in asthma has not been definitively established.
12.2 Pharmacodynamics
In clinical studies with CINQAIR 3 mg/kg, reductions in blood eosinophil counts were observed following the first dose and maintained through 52 weeks of treatment with no signs of tachyphylaxis. Mean eosinophil counts were 696 cells/mcL (n=245) and 624 cells/mcL (n=244) at baseline, and were 55 cells/mcL (92% reduction, n=212) and 496 cells /mcL (21% reduction, n=212) at the Week 52 visit for the CINQAIR and placebo treatment groups, respectively. Early eosinophil reduction was apparent in a subset of patients who had blood eosinophil counts assessed at days 2-3: 220 cells/mcL and 610/mcL for CINQAIR (n=35) and placebo (n=32), respectively. Eosinophils returned towards baseline in those CINQAIR-treated patients who completed a 90-day follow-up assessment (n=35, 480 cells/mcL), approximately 120 days after the last dose of CINQAIR.
Reductions of blood eosinophils were related to reslizumab serum levels, i.e., greater reductions of blood eosinophils were observed in subjects with higher reslizumab serum concentrations.
Treatment-emergent anti-reslizumab antibodies did not interfere with the blood eosinophil reduction effect by reslizumab.
12.3 Pharmacokinetics
The pharmacokinetics (PK) of reslizumab were characterized in healthy adults (n=130), in patients with asthma (n=438), and in other patient populations (n=236). The PK characteristics of reslizumab were similar across these populations. Inter-individual variability in peak and overall exposure was approximately 20-30%.
Peak serum concentrations were typically observed at the end of the infusion. Serum reslizumab concentrations generally declined from peak in a biphasic manner. Following multiple doses, serum concentrations of reslizumab accumulated approximately 1.5 to 1.9-fold.
Systemic exposure to reslizumab appeared to be unaffected by the presence of treatment-emergent anti-reslizumab antibodies.
Distribution
Reslizumab has a volume of distribution of approximately 5 liters, suggesting minimal distribution to the extravascular tissues.
Metabolism
Similar to other monoclonal antibodies, reslizumab is degraded by enzymatic proteolysis into small peptides and amino acids. As reslizumab binds to a soluble target, it is not expected to go through a target-mediated clearance.
Elimination
Reslizumab clearance was approximately 7 mL/hour. Reslizumab has a half-life of about 24 days.
Specific Populations
Age, Race, and Gender
Population PK analyses demonstrated that there was no significant effect of age, race, or gender on the PK of reslizumab.
Hepatic Impairment
No clinical studies were conducted to assess the effect of hepatic impairment on the PK of reslizumab. The results of population PK analyses indicated that there was no significant difference in the PK of reslizumab between patients with normal liver function tests (total bilirubin less than or equal to the ULN and aspartate aminotransferase [AST] less than or equal to the ULN) and mildly increased liver function tests (total bilirubin above the ULN and less than or equal to 1.5-times the ULN or AST greater than ULN and total bilirubin less than or equal to the ULN).
Renal Impairment
No clinical studies have been conducted to assess the effect of renal impairment on the PK of reslizumab. The results of population PK analyses indicated that there was no significant difference in the PK of reslizumab between patients with normal renal function (estimated glomerular filtration rate [eGFR] greater than or equal to 90 mL/min/1.73 m2), mild renal impairment (eGFR 60-89 mL/min/1.73 m2), and moderate renal impairment (eGFR 30-59 mL/min/1.73 m2).
Drug Interactions
In vitro data indicate that IL-5 and reslizumab are unlikely to affect CYP1A2, 2B6, or 3A4 enzyme activity.
No formal clinical drug interaction studies have been conducted with reslizumab. Population PK analyses indicate that concomitant use of either leukotriene antagonists or corticosteroids does not affect the PK of reslizumab.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 6-month bioassay, reslizumab was administered intravenously once every 2 weeks for 26 consecutive weeks (14 total doses) to Tg.rasH2 mice at doses up to 516 mg/kg/dose. There was no evidence of carcinogenicity.
Published literature using animal models suggests that IL-5 and eosinophils are part of an early inflammatory reaction at the site of tumorigenesis and can promote tumor rejection. However, other reports indicate that eosinophil infiltration into tumors can promote tumor growth. Therefore, the malignancy risk in humans from an antibody to IL-5 such as reslizumab is unknown.
In a fertility study, administration of reslizumab to parental mice at doses up to 50 mg/kg (approximately 6 times the MRHD on an AUC basis) had no effects on male or female mating or fertility.
-
14 CLINICAL STUDIES
The asthma development program for CINQAIR 3 mg/kg (administered once every 4 weeks) included 4 randomized, double-blind, placebo-controlled studies (Studies I-IV) 16 to 52 weeks in duration involving 981 patients 12 years of age and older. While patients aged 12 to 17 years were included in these trials, CINQAIR is not approved for use in this age group [see Use in Specific Populations (8.4)]. All subjects continued their background asthma therapy throughout the duration of the studies.
Studies I and II
Studies I and II were 52-week studies in 953 patients with asthma who were required to have a blood eosinophil count of at least 400/mcL (within 3 to 4 weeks of dosing), and at least 1 asthma exacerbation requiring systemic corticosteroid use over the past 12 months. The majority of patients (82%) were on medium-high dose inhaled corticosteroids plus a long-acting beta agonist (ICS/LABA) at baseline. Maintenance oral corticosteroids (OCS) (up to 10 mg of prednisone per day or equivalent) were allowed; 106 (11%) patients were on OCS at baseline. CINQAIR 3 mg/kg administered once every 4 weeks for a total of 13 doses was evaluated compared with placebo.
Study III
Study III was a 16-week study in 315 patients who were required to have a blood eosinophil count of at least 400/mcL at screening (within 3 to 4 weeks of dosing). Maintenance OCS were not allowed. CINQAIR 3 mg/kg or 0.3 mg/kg administered once every 4 weeks for a total of 4 doses was evaluated compared with placebo. While 2 doses of CINQAIR were studied, CINQAIR 3 mg/kg is the only recommended dose [see Dosage and Administration (2.1)].
Study IV
Study IV was a 16-week study in 496 patients unselected for baseline blood eosinophil levels (approximately 80% of patients had a screening [within 3 to 4 weeks of dosing] blood eosinophil count of less than 400/mcL). Maintenance OCS were not allowed. CINQAIR 3 mg/kg administered once every 4 weeks for a total of 4 doses was evaluated compared with placebo.
The demographics and baseline characteristics of these 4 studies is provided in Table 1.
Table 1: Demographics and Baseline Characteristics of Patients in Asthma Studies
Study I
(N=489)Study II
(N=464)Study III
(N=315)Study IV
(N=496)- *
- Baseline for lung function and eosinophil count is the day of randomization.
Mean age (yr)
47
47
44
45
Female (%)
63
63
58
64
White (%)
73
73
81
67
Duration of asthma, mean (yr)
19
18
20
26
Baseline Pre-bronchodilator FEV1, mean % predicted*
64
69
70
67
Baseline Reversibility, mean % ΔFEV1 post-SABA*
26
28
25
26
Baseline mean blood eosinophil count/mcL*
660
649
614
280
Mean number of exacerbations in previous year
1.99
1.94
2.03
1.86
FEV1=forced expiratory volume in 1 second; SABA=short-acting beta agonist.
All patients had to be on inhaled corticosteroid (ICS) background therapy and could have been receiving any combination of background therapies (ICS with or without another controller [non-ICS and/or OCS]).
Exacerbations
The primary endpoint for Studies I and II was the frequency of asthma exacerbations for each patient during the 52-week treatment period. An asthma exacerbation was defined as a worsening of asthma that required at least 1 of the following medical interventions:
1) Either the use of a systemic corticosteroid, or ≥ 2-fold an increase in the use of ICS for 3 or more days, and/or
2) Asthma-related emergency treatment including at least 1 of the following: an unscheduled visit to their healthcare professional for nebulizer treatment or other urgent treatment to prevent worsening of asthma symptoms; a visit to the emergency room for asthma-related treatment; or an asthma-related hospitalization.
The medical intervention had to be corroborated with at least 1 of the following: 1) a decrease in forced expiratory volume in 1 second (FEV1) by 20% or more from baseline, 2) a decrease in peak expiratory flow rate (PEFR) by 30% or more from baseline on 2 consecutive days, or 3) worsening of symptoms or other clinical signs per physician evaluation of the event.
In Studies I and II, patients receiving CINQAIR 3 mg/kg administered once every 4 weeks had significant reductions in the rate of all asthma exacerbations compared to placebo (Table 2). Exacerbations requiring the use of a systemic corticosteroid (e.g., OCS) as well as exacerbations resulting in hospitalization or an emergency room visit were each reduced with CINQAIR 3 mg/kg.
Table 2: Frequency of Asthma Exacerbations during the 52-Week Treatment Period in Patients with Severe Asthma with an Eosinophilic Phenotype (Studies I and II)* Treatment Arm Asthma Exacerbation Rate Rate Ratio
(95% CI)- *
- Randomized patients
All exacerbations
Study I
CINQAIR 3 mg/kg (n=245)
0.90
0.5
(0.37, 0.67)
Placebo (n=244)
1.80
Study II
CINQAIR 3 mg/kg (n=232)
0.86
0.41
(0.28, 0.59)
Placebo (n=232)
2.11
Exacerbations requiring systemic corticosteroid use
Study I
CINQAIR 3 mg/kg (n=245)
0.72
0.45
(0.33, 0.62)
Placebo (n=244)
1.60
Study II
CINQAIR 3 mg/kg (n=232)
0.65
0.39
(0.27, 0.58)
Placebo(n=232)
1.66
Exacerbations resulting in a hospitalization and/or emergency room visit
Study I
CINQAIR 3 mg/kg (n=245)
0.14
0.66
(0.32, 1.36)
Placebo(n=244)
0.21
Study II
CINQAIR 3 mg/kg (n=232)
0.03
0.69
(0.29, 1.65)
Placebo (n=232)
0.05
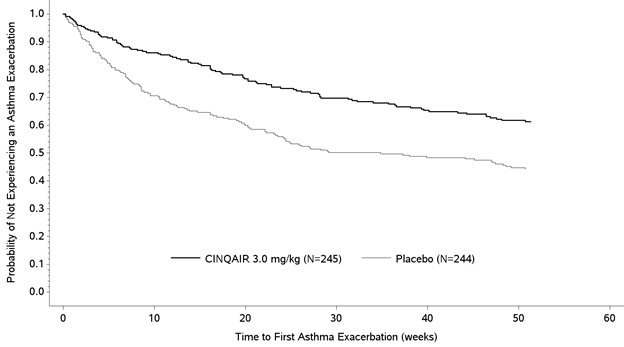
The proportion of patients who did not experience an asthma exacerbation during the 52-week treatment period was higher in the CINQAIR 3 mg/kg group (62% and 75%) compared with the placebo group (46% and 55%), in Studies I and II, respectively. The time to first asthma exacerbation was significantly longer for the groups receiving CINQAIR 3 mg/kg compared with placebo in both Studies I and II. A representative figure from Study I is shown below (Figure 1). Study II showed similar results.
Figure 1: Time to First Asthma Exacerbation by Treatment Group in Patients with Severe Asthma with an Eosinophilic Phenotype (Study I)
Lung Function
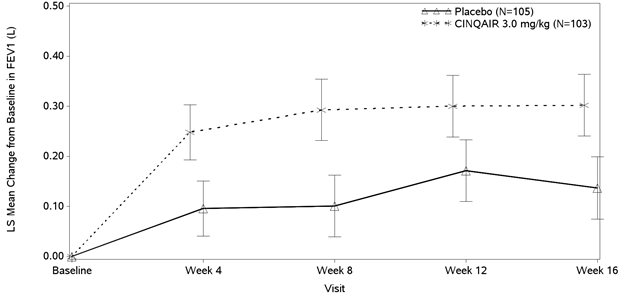
The effect of CINQAIR 3 mg/kg administered once every 4 weeks on FEV1 over time relative to placebo was assessed in all 4 studies (Table 3). FEV1 was the primary endpoint in the 16-week lung function studies: Study III (Figure 2) and Study IV.
Study III also studied a lower dose, CINQAIR 0.3 mg/kg, that produced significant but numerically smaller changes in FEV1 and blood eosinophil reduction compared with the 3 mg/kg dose. While 2 doses of CINQAIR were studied, CINQAIR 3 mg/kg is the only recommended dose [see Dosage and Administration (2.1)].
Study IV was the only study to test CINQAIR 3 mg/kg in asthma patients unselected for blood eosinophils (measured 3 to 4 weeks prior to dosing); association of treatment effect (i.e., difference between CINQAIR and placebo in the change in FEV1 at Week 16) and baseline blood eosinophils was not observed.
Table 3: Mean Change (95% CI) from Baseline in FEV1 in mL Over 16 Weeks (Difference from CINQAIR and Placebo) in Patients with Severe Asthma with an Eosinophilic Phenotype
- *
- Study IV studied asthma patients unselected for blood eosinophils
Study
FEV1 Change in mL
Study I
137 (76, 198)
Study II
93 (30, 155)
Study III
160 (60, 259)
Study IV*
76 (-6,158)
Improvements in FEV1 were observed at 4 weeks following the first dose of CINQAIR for Studies I and II and maintained through Week 52.
Figure 2: Mean Change from Baseline in FEV1 in Patients with Severe Asthma with an Eosinophilic Phenotype (Study III)
The Asthma Control Questionnaire-7 (ACQ-7) and Asthma Quality of Life Questionnaire (AQLQ) were both assessed in Studies I, II, and III. The responder rate for both measures was defined as an improvement in score of 0.5 or more as threshold over 16 weeks.
- For ACQ-7, the responder rate for those randomized to CINQAIR vs. placebo was 69% vs. 65% for Study I, 70% vs. 58% for Study II, and 64% vs. 58% for Study III.
- For AQLQ, the responder rate for those randomized to CINQAIR vs. placebo was 66% vs. 58% for Study I, 67% vs. 55% for Study II, and 64% vs. 48% for Study III.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
CINQAIR (reslizumab) injection, 100 mg/10 mL (10 mg/mL), is supplied as a preservative-free, sterile, clear to slightly hazy/opalescent, colorless to slightly yellow solution in single-use vials. The following packaging configuration is available:
- NDC 59310-610-31: 100 mg/10 mL (10 mg/mL) single-use vial.
Storage and Stability: Refrigerate at 2 ºC to 8ºC (36°F to 46°F). Do not freeze. Do not shake. Protect the vials from light by storing in the original package until time of use.
-
17 PATIENT COUNSELING INFORMATION
See FDA approved patient labeling (Patient Information).
Hypersensitivity/Anaphylaxis
Inform patients that hypersensitivity reactions, including anaphylaxis, have occurred with administration of CINQAIR. Educate patients on the signs and symptoms of hypersensitivity reactions and anaphylaxis (e.g., skin or mucosal involvement, airway compromise, reduced blood pressure). Instruct patients to contact their healthcare professional immediately if they experience symptoms of an allergic reaction after they have received their infusion of CINQAIR [see Warnings and Precautions (5.1)].
Not for Acute Symptoms or Deteriorating Disease
Inform patients that CINQAIR does not treat acute asthma symptoms or acute exacerbations. Inform patients to seek medical advice if their asthma remains uncontrolled or worsens after initiation of treatment with CINQAIR [see Warnings and Precautions (5.2)].
Malignancy
Counsel CINQAIR-treated patients about the risk of malignancies [see Warnings and Precautions (5.3)].
Reduction of Corticosteroid Dosage
Inform patients not to discontinue systemic or inhaled corticosteroids except under the direct supervision of a physician. Inform patients that reduction in corticosteroid dose may be associated with systemic withdrawal symptoms and/or unmask conditions previously suppressed by systemic corticosteroid therapy [see Warnings and Precautions (5.4)].
Manufactured by:
Teva Respiratory, LLC
West Chester, PA 19380
U.S. License Number 2047
US Patent RE39,548 and 6,056,957
©2020 Teva Respiratory, LLC
CNQ-004
-
Patient Package Insert
PATIENT INFORMATION
PATIENT INFORMATION
CINQAIR (sink-ayr’)
(reslizumab) injection for intravenous use
What is the most important information I should know about CINQAIR?
CINQAIR can cause serious side effects, including:
Serious allergic reactions (anaphylaxis). Serious allergic reactions can happen right after you receive your CINQAIR infusion. These reactions can cause death. Allergic reactions sometimes do not happen right away. Your healthcare provider will watch you during and after you receive your CINQAIR infusion for any signs of a reaction. Tell your healthcare provider right away if you have any of the following symptoms that may be associated with an allergic reaction:
• breathing problems • flushing • itching • symptoms of low blood pressure (fainting, dizziness, light headedness, confusion, fast heart beat)
• paleness • skin rash (hives) • swelling of your face, lips, mouth, or tongue • nausea or abdominal discomfort
What is CINQAIR?
CINQAIR is a prescription medicine used with other asthma medicines for the maintenance treatment of asthma in people aged 18 years of age and older whose asthma is not controlled with the current asthma medicines. When added to other medicines for asthma, CINQAIR helps prevent severe asthma attacks (exacerbations) and can improve your breathing. Medicines such as CINQAIR reduce blood eosinophils. Eosinophils are a type of white blood cell that may contribute to your asthma.
- CINQAIR is not used to treat other problems caused by eosinophils.
- CINQAIR is not used to treat sudden breathing problems.
It is not known if CINQAIR is safe and effective in children less than 18 years of age.
Do not receive CINQAIR if you are allergic to reslizumab or any of the ingredients in CINQAIR. See the end of this leaflet for a complete list of ingredients in CINQAIR.
Before receiving CINQAIR, tell your healthcare provider about all of your medical conditions, including if you:
- are taking oral or inhaled corticosteroid medicines. Do not stop taking your corticosteroid unless your healthcare provider tells you to stop. This may cause other symptoms that were controlled by the corticosteroid medicine to come back.
- have or have had cancer (malignancy).
- have a parasitic (helminth) infection.
- are pregnant or plan to become pregnant. It is not known if CINQAIR will harm your unborn baby. Tell your healthcare provider if you become pregnant during your treatment with CINQAIR.
- are breastfeeding or plan to breastfeed. It is not known if CINQAIR passes into your breast milk. You and your healthcare provider should decide if you will receive CINQAIR and breastfeed. Talk to your healthcare provider about the best way to feed your baby if you receive CINQAIR.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Do not stop taking your other asthma medicines unless your healthcare provider tells you to.
How will I receive CINQAIR?
- You will be given CINQAIR by a healthcare provider using a needle placed in a vein (intravenous infusion) 1 time every 4 weeks. It will take about 20 to 50 minutes to receive the full dose of CINQAIR.
What are the possible side effects of CINQAIR?
CINQAIR may cause serious side effects, including:
- See “What is the most important information I should know about CINQAIR?”
- abnormal growth of cells or tissue in your body that may or may not be cancer (malignancy)
The most common side effects of CINQAIR include throat pain.
These are not all the possible side effects of CINQAIR.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of CINQAIR.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use CINQAIR for a condition for which it was not prescribed. You can ask your pharmacist or healthcare provider for information about CINQAIR that is written for healthcare professionals.
What are the ingredients in CINQAIR?
Active ingredient: reslizumab
Inactive ingredients: sodium acetate, acetic acid, sucrose
Manufactured by: Teva Respiratory, LLC; West Chester, PA 19380
U.S. License Number 2047
©2020 Teva Respiratory, LLC
CNQPL-003
For more information, go to www.CINQAIR.com or call 1-888-483-8279.
This Patient Information has been approved by the U.S. Food and Drug Administration
Revised: 02/2020
- Package/Label Display Panel NDC 59310-610-31
- Package/Label Display Panel NDC 59310-610-33
-
INGREDIENTS AND APPEARANCE
CINQAIR
reslizumab injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59310-610 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RESLIZUMAB (UNII: 35A26E427H) (RESLIZUMAB - UNII:35A26E427H) RESLIZUMAB 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) SODIUM ACETATE (UNII: 4550K0SC9B) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59310-610-31 1 in 1 CARTON 04/18/2016 1 10 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 2 NDC:59310-610-33 1 in 1 CARTON 06/04/2017 2 10 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761033 04/18/2016 Labeler - Teva Respiratory, LLC (176697907)