Label: NASAL SPA NATURAL SEA SALT- sodium chloride spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 70176-100-01 - Packager: NACUR HEALTHCARE LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 24, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
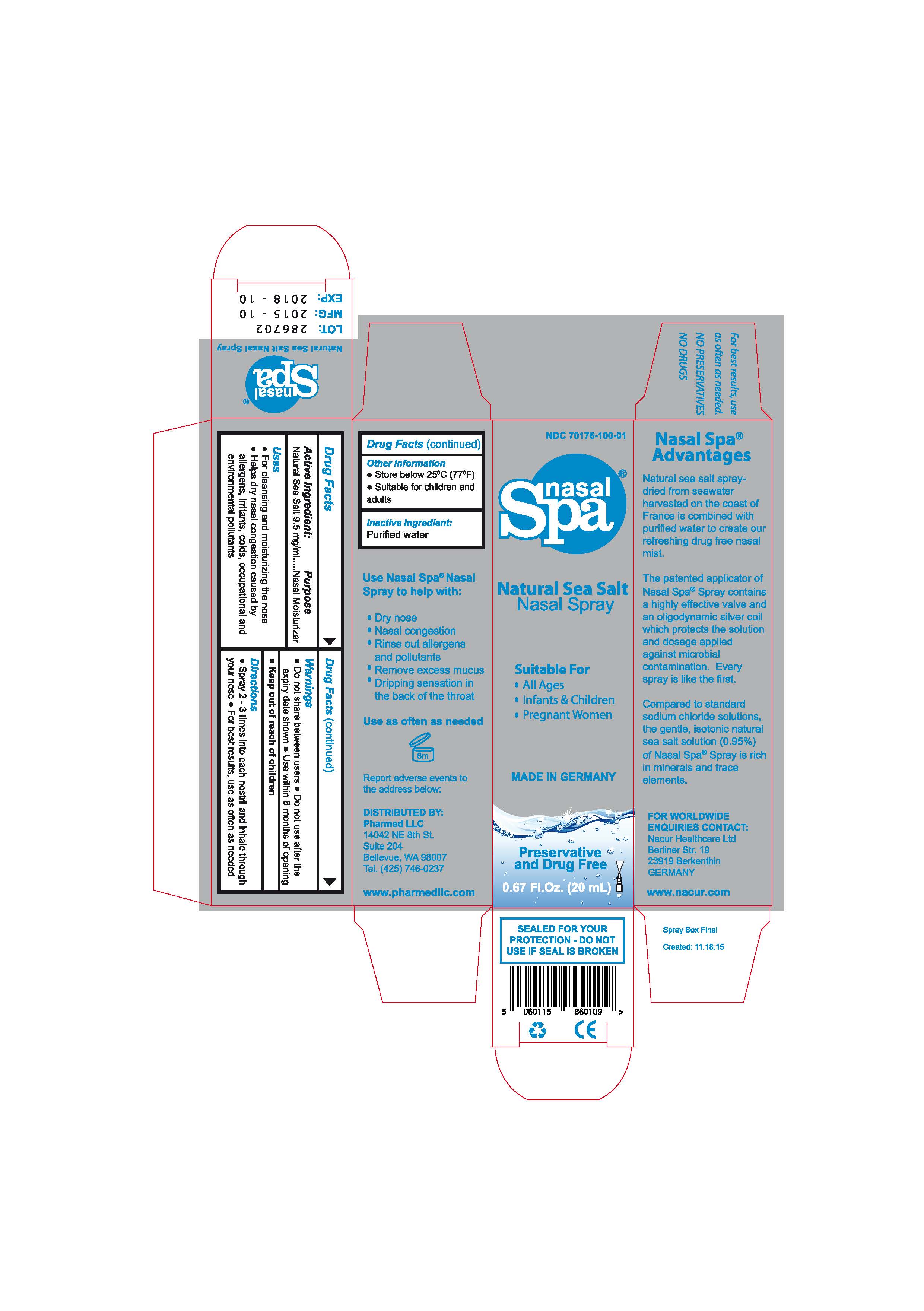
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- DESCRIPTION
- INACTIVE INGREDIENT
- DESCRIPTION
-
DESCRIPTION
The patented applicator of
the Nasal Spa Nasal Spray
contains an highly effective
valve and an oligo-
dynamically active silver coil
which protects the solution
and the dosage applied
against microbial
congestion.
The sea salt is manufactured
in a highly technical
process (spray-drying)
harvesting seawater taken
from an archipelago off
the coast of France in Europe.
Compared to standard
sodium chloride solutions,
the gentle, isotonic natural
sea salt solution (0.95%) of
the Nasal Spa Spray is
rich in minerals and trace
elements.
MADE IN GERMANY
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NASAL SPA NATURAL SEA SALT
sodium chloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70176-100 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 9.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70176-100-01 1 in 1 CARTON 10/29/2015 1 20 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/29/2015 Labeler - NACUR HEALTHCARE LTD (346989317)