Label: ENTRESTO- sacubitril and valsartan tablet, film coated
- NDC Code(s): 71610-807-37
- Packager: Aphena Pharma Solutions - Tennessee, LLC
- This is a repackaged label.
- Source NDC Code(s): 0078-0696
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ENTRESTO safely and effectively. See full prescribing information for ENTRESTO.
ENTRESTO ®(sacubitril and valsartan) tablets, for oral use
Initial U.S. Approval: 2015RECENT MAJOR CHANGES
Indications and Usage, Adult Heart Failure ( 1.1) 2/2021 INDICATIONS AND USAGE
ENTRESTO is indicated:
- to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction (LVEF) below normal. ( 1.1)
- for the treatment of symptomatic heart failure with systemic left ventricular systolic dysfunction in pediatric patients aged one year and older. ENTRESTO reduces NT-proBNP and is expected to improve cardiovascular outcomes. ( 1.2)
DOSAGE AND ADMINISTRATION
Indication Titration Step Dose (twice daily) Starting Second Final Adult Heart Failure 49/51 mg 97/103 mg Pediatric Heart Failure
Patients less than 40 kg1.6 mg/kg 2.3 mg/kg 3.1 mg/kg Pediatric Heart Failure
Patients at least 40 kg, less than 50 kg24/26 mg 49/51 mg 72/78 mg Pediatric Heart Failure
Patients at least 50 kg49/51 mg 72/78 mg 97/103 mg - Adjust adult doses every 2 to 4 weeks and pediatric doses every 2 weeks to the target maintenance dose, as tolerated by the patient. ( 2.2, 2.3)
- Reduce starting dose to half the usually recommended starting dosage for:
- patients not currently taking an ACE inhibitor or ARB or previously taking a low dose of these agents ( 2.5)
- patients with severe renal impairment ( 2.6)
- patients with moderate hepatic impairment ( 2.7)
DOSAGE FORMS AND STRENGTHS
- Film-coated tablets: 24/26 mg; 49/51 mg; 97/103 mg ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Adverse reactions occurring ≥ 5% are hypotension, hyperkalemia, cough, dizziness, and renal failure. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: FETAL TOXICITY
1 INDICATIONS AND USAGE
1.1 Adult Heart Failure
1.2 Pediatric Heart Failure
2 DOSAGE AND ADMINISTRATION
2.1 General Considerations
2.2 Adult Heart Failure
2.3 Pediatric Heart Failure
2.4 Preparation of Oral Suspension
2.5 Dose Adjustment for Patients Not Taking an ACE inhibitor or ARB or Previously Taking Low Doses of These Agents
2.6 Dose Adjustment for Severe Renal Impairment
2.7 Dose Adjustment for Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
5.2 Angioedema
5.3 Hypotension
5.4 Impaired Renal Function
5.5 Hyperkalemia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Dual Blockade of the Renin-Angiotensin-Aldosterone System
7.2 Potassium-Sparing Diuretics
7.3 Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
7.4 Lithium
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Adult Heart Failure
14.2 Pediatric Heart Failure
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- BOXED WARNING (What is this?)
-
1 INDICATIONS AND USAGE
1.1 Adult Heart Failure
ENTRESTO is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in adult patients with chronic heart failure. Benefits are most clearly evident in patients with left ventricular ejection fraction (LVEF) below normal.
LVEF is a variable measure, so use clinical judgment in deciding whom to treat [see Clinical Studies (14.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 General Considerations
ENTRESTO is contraindicated with concomitant use of an angiotensin-converting enzyme (ACE) inhibitor. If switching from an ACE inhibitor to ENTRESTO allow a washout period of 36 hours between administration of the two drugs [see Contraindications (4) and Drug Interactions (7.1)].
2.2 Adult Heart Failure
The recommended starting dose of ENTRESTO is 49/51 mg orally twice-daily.
Double the dose of ENTRESTO after 2 to 4 weeks to the target maintenance dose of 97/103 mg twice daily, as tolerated by the patient.
2.3 Pediatric Heart Failure
Refer to Table 1 for the recommended dose for pediatric patients aged one year and older. Take the recommended dose orally twice daily. Adjust pediatric patient doses every 2 weeks, as tolerated by the patient.
Table 1: Recommended Dose Titration †Use of the Oral Suspension recommended in these patients. Recommended mg/kg doses are of the combined amount of both sacubitril and valsartan [see Dosage and Administration (2.4)].
‡Doses of 72/78 mg can be achieved using three 24/26 mg tablets [see Dosage Forms and Strengths (3)].Titration Step Dose (twice daily) Starting Second Final Pediatric Patients
Less than 40 kg †1.6 mg/kg 2.3 mg/kg 3.1 mg/kg Pediatric Patients
At least 40 kg, less than 50 kg24/26 mg 49/51 mg 72/78 mg ‡ Pediatric Patients
At least 50 kg49/51 mg 72/78 mg ‡ 97/103 mg 2.4 Preparation of Oral Suspension
ENTRESTO oral suspension can be substituted at the recommended tablet dosage in patients unable to swallow tablets.
ENTRESTO 800 mg/200 mL oral suspension can be prepared in a concentration of 4 mg/mL (sacubitril/valsartan 1.96/2.04 mg/mL). Use ENTRESTO 49/51 mg tablets in the preparation of the suspension.
To make an 800 mg/200 mL (4 mg/mL) oral suspension, transfer eight tablets of ENTRESTO 49/51 mg film-coated tablets into a mortar. Crush the tablets into a fine powder using a pestle. Add 60 mL of Ora-Plus ®into the mortar and triturate gently with pestle for 10 minutes, to form a uniform suspension. Add 140 mL of Ora-Sweet ®SF into mortar and triturate with pestle for another 10 minutes, to form a uniform suspension. Transfer the entire contents from the mortar into a clean 200 mL amber colored PET or glass bottle. Place a press-in bottle adapter and close the bottle with a child resistant cap.
The oral suspension can be stored for up to 15 days. Do not store above 25°C (77°F) and do not refrigerate. Shake before each use.
*Ora-Sweet SF ®and Ora-Plus ®are registered trademarks of Paddock Laboratories, Inc.
2.5 Dose Adjustment for Patients Not Taking an ACE inhibitor or ARB or Previously Taking Low Doses of These Agents
In patients not currently taking an ACE inhibitor or an angiotensin II receptor blocker (ARB) and for patients previously taking low doses of these agents, start ENTRESTO at half the usually recommended starting dose. After initiation, increase the dose every 2 to 4 weeks in adults and every 2 weeks in pediatric patients to follow the recommended dose escalation thereafter [see Dosage and Administration (2.2, 2.3)].
Note: Initiate pediatric patients weighing 40 to 50 kg who meet this criterion at 0.8 mg/kg twice daily using the oral suspension [see Dosage and Administration (2.3, 2.4)].
2.6 Dose Adjustment for Severe Renal Impairment
In adults and pediatric patients with severe renal impairment (eGFR < 30 mL/min/1.73 m 2), start ENTRESTO at half the usually recommended starting dose. After initiation, increase the dose to follow the recommended dose escalation thereafter [see Dosage and Administration (2.2, 2.3)].
Note: Initiate pediatric patients weighing 40 to 50 kg who meet this criterion at 0.8 mg/kg twice daily using the oral suspension [see Dosage and Administration (2.3, 2.4)].
No starting dose adjustment is needed for mild or moderate renal impairment.
2.7 Dose Adjustment for Hepatic Impairment
In adults and pediatric patients with moderate hepatic impairment (Child-Pugh B classification), start ENTRESTO at half the usually recommended starting dose. After initiation, increase the dose to follow the recommended dose escalation thereafter [see Dosage and Administration (2.2, 2.3)].
Note: Initiate pediatric patients weighing 40 to 50 kg who meet this criterion at 0.8 mg/kg twice daily using the oral suspension [see Dosage and Administration (2.3, 2.4)].
No starting dose adjustment is needed for mild hepatic impairment.
Use in patients with severe hepatic impairment is not recommended.
-
3 DOSAGE FORMS AND STRENGTHS
ENTRESTO is supplied as unscored, ovaloid, film-coated tablets in the following strengths:
ENTRESTO 24/26 mg, (sacubitril 24 mg and valsartan 26 mg) are violet white and debossed with “NVR” on one side and “LZ” on the other side.
ENTRESTO 49/51 mg, (sacubitril 49 mg and valsartan 51 mg) are pale yellow and debossed with “NVR” on one side and “L1” on the other side.
ENTRESTO 97/103 mg, (sacubitril 97 mg and valsartan 103 mg) are light pink and debossed with “NVR” on one side and “L11” on the other side.
-
4 CONTRAINDICATIONS
ENTRESTO is contraindicated:
- in patients with hypersensitivity to any component
- in patients with a history of angioedema related to previous ACE inhibitor or ARB therapy [see Warnings and Precautions (5.2)]
- with concomitant use of ACE inhibitors. Do not administer within 36 hours of switching from or to an ACE inhibitor [see Drug Interactions (7.1)]
- with concomitant use of aliskiren in patients with diabetes [see Drug Interactions (7.1)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
ENTRESTO can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. When pregnancy is detected, consider alternative drug treatment and discontinue ENTRESTO. However, if there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system, and if the drug is considered lifesaving for the mother, advise a pregnant woman of the potential risk to the fetus [see Use in Specific Populations (8.1)].
5.2 Angioedema
ENTRESTO may cause angioedema [see Adverse Reactions (6.1)]. If angioedema occurs, discontinue ENTRESTO immediately, provide appropriate therapy, and monitor for airway compromise. ENTRESTO must not be re-administered. In cases of confirmed angioedema where swelling has been confined to the face and lips, the condition has generally resolved without treatment, although antihistamines have been useful in relieving symptoms.
Angioedema associated with laryngeal edema may be fatal. Where there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, administer appropriate therapy, e.g., subcutaneous epinephrine/adrenaline solution 1:1000 (0.3 mL to 0.5 mL) and take measures necessary to ensure maintenance of a patent airway.
ENTRESTO has been associated with a higher rate of angioedema in Black than in non-Black patients.
Patients with a prior history of angioedema may be at increased risk of angioedema with ENTRESTO [see Adverse Reactions (6.1)]. ENTRESTO must not be used in patients with a known history of angioedema related to previous ACE inhibitor or ARB therapy [see Contraindications (4)]. ENTRESTO should not be used in patients with hereditary angioedema.
5.3 Hypotension
ENTRESTO lowers blood pressure and may cause symptomatic hypotension [see Adverse Reactions (6.1)]. Patients with an activated renin-angiotensin system, such as volume- and/or salt-depleted patients (e.g., those being treated with high doses of diuretics), are at greater risk. Correct volume or salt depletion prior to administration of ENTRESTO or start at a lower dose. If hypotension occurs, consider dose adjustment of diuretics, concomitant antihypertensive drugs, and treatment of other causes of hypotension (e.g., hypovolemia). If hypotension persists despite such measures, reduce the dosage or temporarily discontinue ENTRESTO. Permanent discontinuation of therapy is usually not required.
5.4 Impaired Renal Function
As a consequence of inhibiting the renin-angiotensin-aldosterone system (RAAS), decreases in renal function may be anticipated in susceptible individuals treated with ENTRESTO [see Adverse Reactions (6.1)]. In patients whose renal function depends upon the activity of the renin-angiotensin-aldosterone system (e.g., patients with severe congestive heart failure), treatment with ACE inhibitors and angiotensin receptor antagonists has been associated with oliguria, progressive azotemia and, rarely, acute renal failure and death. Closely monitor serum creatinine, and down-titrate or interrupt ENTRESTO in patients who develop a clinically significant decrease in renal function [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
As with all drugs that affect the RAAS, ENTRESTO may increase blood urea and serum creatinine levels in patients with bilateral or unilateral renal artery stenosis. In patients with renal artery stenosis, monitor renal function.
5.5 Hyperkalemia
Through its actions on the RAAS, hyperkalemia may occur with ENTRESTO [see Adverse Reactions (6.1)]. Monitor serum potassium periodically and treat appropriately, especially in patients with risk factors for hyperkalemia such as severe renal impairment, diabetes, hypoaldosteronism, or a high potassium diet. Dosage reduction or interruption of ENTRESTO may be required [see Dosage and Administration (2.6)].
-
6 ADVERSE REACTIONS
Clinically significant adverse reactions that appear in other sections of the labeling include:
- Angioedema [see Warnings and Precautions (5.2)]
- Hypotension [see Warnings and Precautions (5.3)]
- Impaired Renal Function [see Warnings and Precautions (5.4)]
- Hyperkalemia [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 6,622 heart failure patients were treated with ENTRESTO in the PARADIGM-HF (vs. enalapril) and PARAGON-HF (vs. valsartan) clinical trials. Of these, 5,085 were exposed for at least 1 year.
Adult Heart Failure
In PARADIGM-HF, patients were required to complete sequential enalapril and ENTRESTO run-in periods of (median) 15 and 29 days, respectively, prior to entering the randomized double-blind period comparing ENTRESTO and enalapril. During the enalapril run-in period, 1,102 patients (10.5%) were permanently discontinued from the study, 5.6% because of an adverse event, most commonly renal dysfunction (1.7%), hyperkalemia (1.7%) and hypotension (1.4%). During the ENTRESTO run-in period, an additional 10.4% of patients permanently discontinued treatment, 5.9% because of an adverse event, most commonly renal dysfunction (1.8%), hypotension (1.7%) and hyperkalemia (1.3%). Because of this run-in design, the adverse reaction rates described below are lower than expected in practice.
In the double-blind period, safety was evaluated in 4,203 patients treated with ENTRESTO and 4,229 treated with enalapril. In PARADIGM-HF, patients randomized to ENTRESTO received treatment for up to 4.3 years, with a median duration of exposure of 24 months; 3,271 patients were treated for more than one year. Discontinuation of therapy because of an adverse event during the double-blind period occurred in 450 (10.7%) of ENTRESTO treated patients and 516 (12.2%) of patients receiving enalapril.
Adverse reactions occurring at an incidence of ≥ 5% in patients who were treated with ENTRESTO in the double-blind period of PARADIGM-HF are shown in Table 2.
In PARADIGM-HF, the incidence of angioedema was 0.1% in both the enalapril and ENTRESTO run-in periods. In the double-blind period, the incidence of angioedema was higher in patients treated with ENTRESTO than enalapril (0.5% and 0.2%, respectively). The incidence of angioedema in Black patients was 2.4% with ENTRESTO and 0.5% with enalapril [see Warnings and Precautions (5.2)].
Orthostasis was reported in 2.1% of patients treated with ENTRESTO compared to 1.1% of patients treated with enalapril during the double-blind period of PARADIGM-HF. Falls were reported in 1.9% of patients treated with ENTRESTO compared to 1.3% of patients treated with enalapril.
Table 2: Adverse Reactions Reported in ≥ 5% of Patients Treated with ENTRESTO in the Double-Blind Period of PARADIGM-HF ENTRESTO
(n = 4,203)
%Enalapril
(n = 4,229)
%Hypotension 18 12 Hyperkalemia 12 14 Cough 9 13 Dizziness 6 5 Renal failure/acute renal failure 5 5 In PARAGON-HF, no new adverse reactions were identified.
Pediatric Heart Failure
The adverse reactions observed in pediatric patients 1 to < 18 years old who received treatment with ENTRESTO were consistent with those observed in adult patients.
Laboratory Abnormalities
Hemoglobin and Hematocrit
Decreases in hemoglobin/hematocrit of > 20% were observed in approximately 5% of both ENTRESTO- and enalapril-treated patients in the double-blind period in PARADIGM-HF. Decreases in hemoglobin/hematocrit of >20% were observed in approximately 7% of ENTRESTO-treated patients and 9% of valsartan-treated patients in the double-blind period in PARAGON-HF.
Serum Creatinine
During the double-blind period in PARADIGM-HF, approximately 16% of both ENTRESTO- and enalapril-treated patients had increases in serum creatinine of > 50%. During the double-blind period in PARAGON-HF, approximately 17% of ENTRESTO-treated patients and 21% of valsartan-treated patients had increases in serum creatinine of > 50%.
Serum Potassium
During the double-blind period of PARADIGM-HF, approximately 16% of both ENTRESTO- and enalapril-treated patients had potassium concentrations > 5.5 mEq/L. During the double-blind period of PARAGON-HF, approximately 18% of ENTRESTO-treated patients and 20% of valsartan-treated patients had potassium concentrations > 5.5 mEq/L.
6.2 Postmarketing Experience
The following additional adverse reactions have been reported in postmarketing experience. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity including rash, pruritus, and anaphylactic reaction
-
7 DRUG INTERACTIONS
7.1 Dual Blockade of the Renin-Angiotensin-Aldosterone System
Concomitant use of ENTRESTO with an ACE inhibitor is contraindicated because of the increased risk of angioedema [see Contraindications (4)].
Avoid use of ENTRESTO with an ARB, because ENTRESTO contains the angiotensin II receptor blocker valsartan.
The concomitant use of ENTRESTO with aliskiren is contraindicated in patients with diabetes [see Contraindications (4)]. Avoid use with aliskiren in patients with renal impairment (eGFR < 60 mL/min/1.73 m 2).
7.2 Potassium-Sparing Diuretics
As with other drugs that block angiotensin II or its effects, concomitant use of potassium-sparing diuretics (e.g., spironolactone, triamterene, amiloride), potassium supplements, or salt substitutes containing potassium may lead to increases in serum potassium [see Warnings and Precautions (5.5)].
7.3 Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, concomitant use of NSAIDs, including COX-2 inhibitors, with ENTRESTO may result in worsening of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
ENTRESTO can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. In animal reproduction studies, ENTRESTO treatment during organogenesis resulted in increased embryo-fetal lethality in rats and rabbits and teratogenicity in rabbits. When pregnancy is detected, consider alternative drug treatment and discontinue ENTRESTO. However, if there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system, and if the drug is considered lifesaving for the mother, advise a pregnant woman of the potential risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Oligohydramnios in pregnant women who use drugs affecting the renin-angiotensin system in the second and third trimesters of pregnancy can result in the following: reduced fetal renal function leading to anuria and renal failure, fetal lung hypoplasia, skeletal deformations, including skull hypoplasia, hypotension, and death.
Perform serial ultrasound examinations to assess the intra-amniotic environment. Fetal testing may be appropriate, based on the week of gestation. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. If oligohydramnios is observed, consider alternative drug treatment. Closely observe neonates with histories of in uteroexposure to ENTRESTO for hypotension, oliguria, and hyperkalemia. In neonates with a history of in uteroexposure to ENTRESTO, if oliguria or hypotension occurs, support blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and replacing renal function.
Data
Animal Data
ENTRESTO treatment during organogenesis resulted in increased embryo-fetal lethality in rats at doses ≥ 49 mg sacubitril/51 mg valsartan/kg/day (≤ 0.06 [LBQ657, the active metabolite] and 0.72 [valsartan]-fold the maximum recommended human dose [MRHD] of 97/103 mg twice-daily on the basis of the area under the plasma drug concentration-time curve [AUC]) and rabbits at doses ≥ 5 mg sacubitril/5 mg valsartan/kg/day (2-fold and 0.03-fold the MRHD on the basis of valsartan and LBQ657 AUC, respectively). ENTRESTO is teratogenic based on a low incidence of fetal hydrocephaly, associated with maternally toxic doses, which was observed in rabbits at an ENTRESTO dose of ≥ 5 mg sacubitril/5 mg valsartan/kg/day. The adverse embryo-fetal effects of ENTRESTO are attributed to the angiotensin receptor antagonist activity.
Pre- and postnatal development studies in rats at sacubitril doses up to 750 mg/kg/day (2.2-fold the MRHD on the basis of LBQ657 AUC) and valsartan at doses up to 600 mg/kg/day (0.86-fold the MRHD on the basis of AUC) indicate that treatment with ENTRESTO during organogenesis, gestation and lactation may affect pup development and survival.
8.2 Lactation
Risk Summary
There is no information regarding the presence of sacubitril/valsartan in human milk, the effects on the breastfed infant, or the effects on milk production. Sacubitril/valsartan is present in rat milk. Because of the potential for serious adverse reactions in breastfed infants from exposure to sacubitril/valsartan, advise a nursing woman that breastfeeding is not recommended during treatment with ENTRESTO.
Data
Following an oral dose (15 mg sacubitril/15 mg valsartan/kg) of [ 14C] ENTRESTO to lactating rats, transfer of LBQ657 into milk was observed. After a single oral administration of 3 mg/kg [ 14C] valsartan to lactating rats, transfer of valsartan into milk was observed.
8.4 Pediatric Use
The safety and effectiveness of ENTRESTO in pediatric heart failure patients 1 to < 18 years old are supported by the reduction from baseline to 12 weeks in NT-proBNP in a randomized, double-blind clinical study [see Clinical Studies (14.2)]. The analysis of NT-proBNP included 90 patients age 6 to 18 years and 20 patients age 1 to 6 years.
Safety and effectiveness have not been established in pediatric patients less than 1 year of age.
Animal Data
Sacubitril given orally to juvenile rats from postnatal day (PND) 7 to PND 35 or PND 70 (an age approximately equivalent to neonatal through pre-pubertal development or adulthood in humans) at doses ≥ 400 mg/kg/day (approximately 2-fold the AUC exposure to the active metabolite of sacubitril, LBQ657, at an ENTRESTO pediatric clinical dose of 3.1 mg/kg twice daily) resulted in decreases in body weight, bone length, and bone mass. The decrease in body weight was transient from PND 10 to PND 20 and the effects for most bone parameters were reversible after treatment stopped. Exposure at the No-Observed-Adverse-Effect-Level (NOAEL) of 100 mg/kg/day was approximately 0.5-fold the AUC exposure to LBQ657 at the 3.1 mg/kg twice daily dose of ENTRESTO. The mechanism underlying bone effects in rats and the translatability to pediatric patients are unknown.
Valsartan given orally to juvenile rats from PND 7 to PND 70 (an age approximately equivalent to neonatal through adulthood in humans) produced persistent, irreversible kidney damage at all dose levels. Exposure at the lowest tested dose of 1 mg/kg/day was approximately 0.2-fold the exposure at 3.1 mg/kg twice daily dose of ENTRESTO based on AUC. These kidney effects in neonatal rats represent expected exaggerated pharmacological effects that are observed if rats are treated during the first 13 days of life.
8.5 Geriatric Use
No relevant pharmacokinetic differences have been observed in elderly (≥ 65 years) or very elderly (≥ 75 years) patients compared to the overall population [see Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
No dose adjustment is required when administering ENTRESTO to patients with mild hepatic impairment (Child-Pugh A classification). The recommended starting dose in patients with moderate hepatic impairment (Child-Pugh B classification) is 24/26 mg twice daily. The use of ENTRESTO in patients with severe hepatic impairment (Child-Pugh C classification) is not recommended, as no studies have been conducted in these patients [see Dosage and Administration (2.6), Clinical Pharmacology (12.3)].
8.7 Renal Impairment
No dose adjustment is required in patients with mild (eGFR 60 to 90 mL/min/1.73 m 2) to moderate (eGFR 30 to 60 mL/min/1.73 m 2) renal impairment. The recommended starting dose in patients with severe renal impairment (eGFR < 30 mL/min/1.73 m 2) is 24/26 mg twice daily [see Dosage and Administration (2.5), Warnings and Precautions (5.4), and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Limited data are available with regard to overdosage in human subjects with ENTRESTO. In healthy volunteers, a single dose of ENTRESTO 583 mg sacubitril/617 mg valsartan, and multiple doses of 437 mg sacubitril/463 mg valsartan (14 days) have been studied and were well tolerated.
Hypotension is the most likely result of overdosage due to the blood pressure lowering effects of ENTRESTO. Symptomatic treatment should be provided.
ENTRESTO is unlikely to be removed by hemodialysis because of high protein binding.
-
11 DESCRIPTION
ENTRESTO (sacubitril and valsartan) is a combination of a neprilysin inhibitor and an angiotensin II receptor blocker.
ENTRESTO contains a complex comprised of anionic forms of sacubitril and valsartan, sodium cations, and water molecules in the molar ratio of 1:1:3:2.5, respectively. Following oral administration, the complex dissociates into sacubitril (which is further metabolized to LBQ657) and valsartan. The complex is chemically described as Octadecasodiumhexakis(4-{[(1S,3R)-1-([1,1´-biphenyl]-4-ylmethyl)-4-ethoxy-3-methyl-4-oxobutyl]amino}-4-oxobutanoate)hexakis(N-pentanoyl-N-{[2´-(1H-tetrazol-1-id-5-yl)[1,1´-biphenyl]-4-yl]methyl}-L-valinate)—water (1/15).
Its empirical formula (hemipentahydrate) is C 48H 55N 6O 8Na 32.5 H 2O. Its molecular mass is 957.99 and its schematic structural formula is:
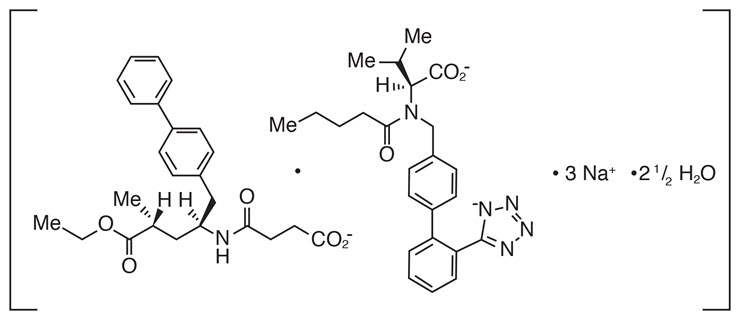
ENTRESTO is available as film-coated tablets for oral administration, containing 24 mg of sacubitril and 26 mg of valsartan; 49 mg of sacubitril and 51 mg of valsartan; and 97 mg of sacubitril and 103 mg of valsartan. The tablet inactive ingredients are microcrystalline cellulose, low-substituted hydroxypropylcellulose, crospovidone, magnesium stearate (vegetable origin), talc, and colloidal silicon dioxide. The film-coat inactive ingredients are hypromellose, titanium dioxide (E 171), Macrogol 4000, talc, and iron oxide red (E 172). The film-coat for the 24 mg of sacubitril and 26 mg of valsartan tablet and the 97 mg of sacubitril and 103 mg of valsartan tablet also contains iron oxide black (E 172). The film-coat for the 49 mg of sacubitril and 51 mg of valsartan tablet contains iron oxide yellow (E 172).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ENTRESTO contains a neprilysin inhibitor, sacubitril, and an angiotensin receptor blocker, valsartan. ENTRESTO inhibits neprilysin (neutral endopeptidase; NEP) via LBQ657, the active metabolite of the prodrug sacubitril, and blocks the angiotensin II type-1 (AT 1) receptor via valsartan. The cardiovascular and renal effects of ENTRESTO in heart failure patients are attributed to the increased levels of peptides that are degraded by neprilysin, such as natriuretic peptides, by LBQ657, and the simultaneous inhibition of the effects of angiotensin II by valsartan. Valsartan inhibits the effects of angiotensin II by selectively blocking the AT 1receptor, and also inhibits angiotensin II-dependent aldosterone release.
12.2 Pharmacodynamics
The pharmacodynamic effects of ENTRESTO were evaluated after single and multiple dose administrations in healthy subjects and in patients with heart failure, and are consistent with simultaneous neprilysin inhibition and renin-angiotensin system blockade.
In a 7-day valsartan-controlled study in patients with reduced ejection fraction (HFrEF), administration of ENTRESTO resulted in a significant non-sustained increase in natriuresis, increased urine cGMP, and decreased plasma MR-proANP and NT-proBNP compared to valsartan.
In a 21-day study in HFrEF patients, ENTRESTO significantly increased urine ANP and cGMP and plasma cGMP, and decreased plasma NT-proBNP, aldosterone and endothelin-1. ENTRESTO also blocked the AT 1-receptor as evidenced by increased plasma renin activity and plasma renin concentrations. In PARADIGM-HF, ENTRESTO decreased plasma NT-proBNP (not a neprilysin substrate) and increased plasma BNP (a neprilysin substrate) and urine cGMP compared with enalapril.
In PARAMOUNT, a randomized, double-blind, 36-week study in patients with heart failure with LVEF ≥ 45% comparing 97/103 mg of ENTRESTO (n=149) to 160 mg of valsartan (n =152) twice-daily, ENTRESTO decreased NT-proBNP by 17% while valsartan increased NT-proBNP by 8% at Week 12 (p = 0.005).
In PARAGON-HF, ENTRESTO decreased NT-proBNP by 24% (Week 16) and 19% (Week 48) compared to 6% and 3% reductions on valsartan, respectively.
QT Prolongation: In a thorough QTc clinical study in healthy male subjects, single doses of ENTRESTO 194 mg sacubitril/206 mg valsartan and 583 mg sacubitril/617 mg valsartan had no effect on cardiac repolarization.
Amyloid-β: Neprilysin is one of multiple enzymes involved in the clearance of amyloid-β (Aβ) from the brain and cerebrospinal fluid (CSF). Administration of ENTRESTO 194 mg sacubitril/206 mg valsartan once-daily for 2 weeks to healthy subjects was associated with an increase in CSF Aβ 1-38compared to placebo; there were no changes in concentrations of CSF Aβ 1-40or CSF Aβ 1-42. The clinical relevance of this finding is unknown [see Nonclinical Toxicology (13)].
Blood Pressure: Addition of a 50 mg single dose of sildenafil to ENTRESTO at steady state (194 mg sacubitril/206 mg valsartan once daily for 5 days) in patients with hypertension was associated with additional blood pressure (BP) reduction (~ 5/4 mmHg, systolic/diastolic BP) compared to administration of ENTRESTO alone.
Co-administration of ENTRESTO did not significantly alter the BP effect of intravenous nitroglycerin.
12.3 Pharmacokinetics
Absorption
Following oral administration, ENTRESTO dissociates into sacubitril and valsartan. Sacubitril is further metabolized to LBQ657. The peak plasma concentrations of sacubitril, LBQ657, and valsartan are reached in 0.5 hours, 2 hours, and 1.5 hours, respectively. The oral absolute bioavailability of sacubitril is estimated to be ≥ 60%. The valsartan in ENTRESTO is more bioavailable than the valsartan in other marketed tablet formulations; 26 mg, 51 mg, and 103 mg of valsartan in ENTRESTO is equivalent to 40 mg, 80 mg, and 160 mg of valsartan in other marketed tablet formulations, respectively.
Following twice-daily dosing of ENTRESTO, steady state levels of sacubitril, LBQ657, and valsartan are reached in 3 days. At steady state, sacubitril and valsartan do not accumulate significantly, whereas LBQ657 accumulates by 1.6-fold. ENTRESTO administration with food has no clinically significant effect on the systemic exposures of sacubitril, LBQ657, or valsartan. Although there is a decrease in exposure to valsartan when ENTRESTO is administered with food, this decrease is not accompanied by a clinically significant reduction in the therapeutic effect. ENTRESTO can therefore be administered with or without food.
Distribution
Sacubitril, LBQ657 and valsartan are highly bound to plasma proteins (94% to 97%). Based on the comparison of plasma and CSF exposures, LBQ657 crosses the blood brain barrier to a limited extent (0.28%). The average apparent volumes of distribution of valsartan and sacubitril are 75 and 103 L, respectively.
Metabolism
Sacubitril is readily converted to LBQ657 by esterases; LBQ657 is not further metabolized to a significant extent. Valsartan is minimally metabolized; only about 20% of the dose is recovered as metabolites. A hydroxyl metabolite has been identified in plasma at low concentrations (< 10%).
Elimination
Following oral administration, 52% to 68% of sacubitril (primarily as LBQ657) and ~ 13% of valsartan and its metabolites are excreted in urine; 37% to 48% of sacubitril (primarily as LBQ657), and 86% of valsartan and its metabolites are excreted in feces. Sacubitril, LBQ657, and valsartan are eliminated from plasma with a mean elimination half-life (T 1/2) of approximately 1.4 hours, 11.5 hours, and 9.9 hours, respectively.
Linearity/Nonlinearity
The pharmacokinetics of sacubitril, LBQ657, and valsartan were linear over an ENTRESTO dose range of 24 mg sacubitril/26 mg valsartan to 194 mg sacubitril/206 mg valsartan.
Drug Interactions:
Effect of Co-administered Drugs on ENTRESTO:
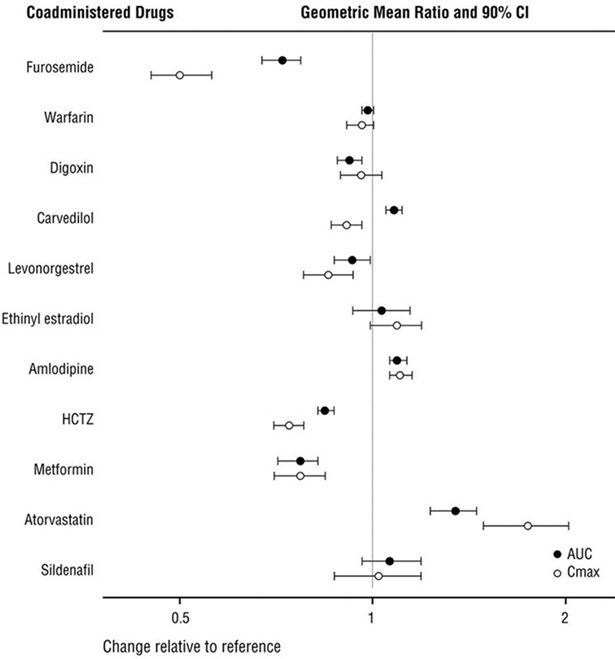
Because CYP450 enzyme-mediated metabolism of sacubitril and valsartan is minimal, coadministration with drugs that impact CYP450 enzymes is not expected to affect the pharmacokinetics of ENTRESTO. Dedicated drug interaction studies demonstrated that coadministration of furosemide, warfarin, digoxin, carvedilol, a combination of levonorgestrel/ethinyl estradiol, amlodipine, omeprazole, hydrochlorothiazide (HCTZ), metformin, atorvastatin, and sildenafil, did not alter the systemic exposure to sacubitril, LBQ657 or valsartan.
Effect of ENTRESTO on Co-administered Drugs:
In vitrodata indicate that sacubitril inhibits OATP1B1 and OATP1B3 transporters. The effects of ENTRESTO on the pharmacokinetics of coadministered drugs are summarized in Figure 1.
Figure 1: Effect of ENTRESTO on Pharmacokinetics of Coadministered Drugs
Specific Populations
Effect of specific populations on the pharmacokinetics of LBQ657 and valsartan are shown in Figure 2.
Figure 2: Pharmacokinetics of ENTRESTO in Specific Populations
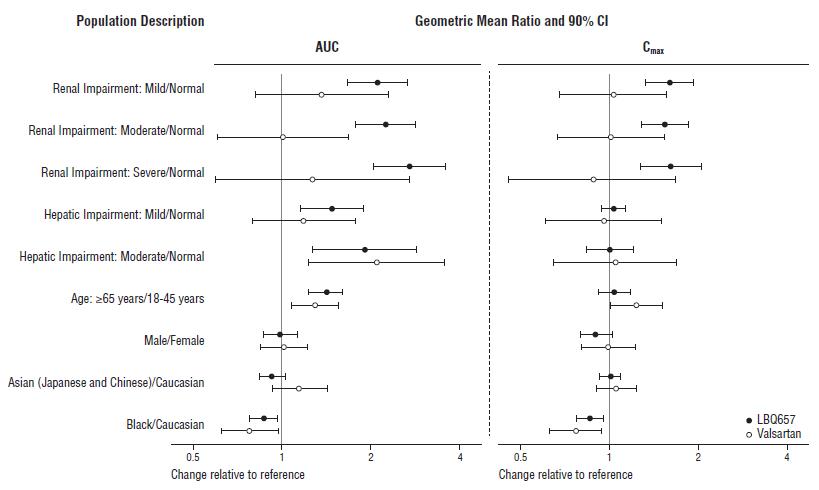
Note: Child-Pugh Classification was used for hepatic impairment.
Pediatric Patients:
The pharmacokinetics of ENTRESTO were evaluated in pediatric heart failure patients 1 to < 18 years old administered oral doses of 0.8 mg/kg and 3.1 mg/kg of ENTRESTO. Pharmacokinetic data indicated that exposure to ENTRESTO in pediatric and adult patients is similar.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
Carcinogenicity studies conducted in mice and rats with sacubitril and valsartan did not identify any carcinogenic potential for ENTRESTO. The LBQ657 C maxat the high dose (HD) of 1200 mg/kg/day in male and female mice was, respectively, 14 and 16 times that in humans at the MRHD. The LBQ657 C maxin male and female rats at the HD of 400 mg/kg/day was, respectively, 1.7 and 3.5 times that at the MRHD. The doses of valsartan studied (high dose of 160 and 200 mg/kg/day in mice and rats, respectively) were about 4 and 10 times, respectively, the MRHD on a mg/m 2basis.
Mutagenicity and clastogenicity studies conducted with ENTRESTO, sacubitril, and valsartan did not reveal any effects at either the gene or chromosome level.
Impairment of Fertility
ENTRESTO did not show any effects on fertility in rats up to a dose of 73 mg sacubitril/77 mg valsartan/kg/day (≤ 1.0-fold and ≤ 0.18-fold the MRHD on the basis of the AUCs of valsartan and LBQ657, respectively).
13.2 Animal Toxicology and/or Pharmacology
The effects of ENTRESTO on amyloid-β concentrations in CSF and brain tissue were assessed in young (2 to 4 years old) cynomolgus monkeys treated with ENTRESTO (24 mg sacubitril/26 mg valsartan/kg/day) for 2 weeks. In this study, ENTRESTO affected CSF Aβ clearance, increasing CSF Aβ 1-40, 1-42, and 1-38 levels in CSF; there was no corresponding increase in Aβ levels in the brain. In addition, in a toxicology study in cynomolgus monkeys treated with ENTRESTO at 146 mg sacubitril/154 mg valsartan/kg/day for 39-weeks, there was no amyloid-β accumulation in the brain.
-
14 CLINICAL STUDIES
Dosing in clinical trials was based on the total amount of both components of ENTRESTO, i.e., 24/26 mg, 49/51 mg, and 97/103 mg were referred to as 50 mg, 100 mg, and 200 mg, respectively.
14.1 Adult Heart Failure
PARADIGM-HF
PARADIGM-HF was a multinational, randomized, double-blind trial comparing ENTRESTO and enalapril in 8,442 adult patients with symptomatic chronic heart failure (NYHA class II–IV) and systolic dysfunction (left ventricular ejection fraction ≤ 40%). Patients had to have been on an ACE inhibitor or ARB for at least four weeks and on maximally tolerated doses of beta-blockers. Patients with a systolic blood pressure of < 100 mmHg at screening were excluded.
The primary objective of PARADIGM-HF was to determine whether ENTRESTO, a combination of sacubitril and a RAS inhibitor (valsartan), was superior to a RAS inhibitor (enalapril) alone in reducing the risk of the combined endpoint of cardiovascular (CV) death or hospitalization for heart failure (HF).
After discontinuing their existing ACE inhibitor or ARB therapy, patients entered sequential single-blind run-in periods during which they received enalapril 10 mg twice-daily, followed by ENTRESTO 100 mg twice-daily, increasing to 200 mg twice-daily. Patients who successfully completed the sequential run-in periods were randomized to receive either ENTRESTO 200 mg (N = 4,209) twice-daily or enalapril 10 mg (N = 4,233) twice-daily. The primary endpoint was the first event in the composite of CV death or hospitalization for HF. The median follow-up duration was 27 months and patients were treated for up to 4.3 years.
The population was 66% Caucasian, 18% Asian, and 5% Black; the mean age was 64 years and 78% were male. At randomization, 70% of patients were NYHA Class II, 24% were NYHA Class III, and 0.7% were NYHA Class IV. The mean left ventricular ejection fraction was 29%. The underlying cause of heart failure was coronary artery disease in 60% of patients; 71% had a history of hypertension, 43% had a history of myocardial infarction, 37% had an eGFR < 60 mL/min/1.73m 2, and 35% had diabetes mellitus. Most patients were taking beta-blockers (94%), mineralocorticoid antagonists (58%), and diuretics (82%). Few patients had an implantable cardioverter-defibrillator (ICD) or cardiac resynchronization therapy-defibrillator (CRT-D) (15%).
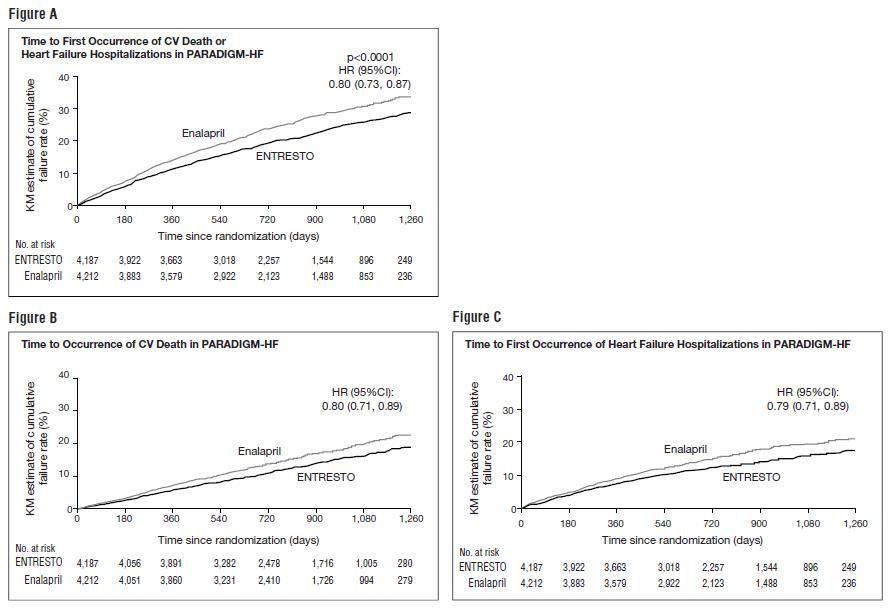
PARADIGM-HF demonstrated that ENTRESTO, a combination of sacubitril and a RAS inhibitor (valsartan), was superior to a RAS inhibitor (enalapril), in reducing the risk of the combined endpoint of cardiovascular death or hospitalization for heart failure, based on a time-to-event analysis (hazard ratio [HR] 0.80; 95% confidence interval [CI], 0.73, 0.87, p< 0.0001). The treatment effect reflected a reduction in both cardiovascular death and heart failure hospitalization; see Table 3 and Figure 3. Sudden death accounted for 45% of cardiovascular deaths, followed by pump failure, which accounted for 26%.
ENTRESTO also improved overall survival (HR 0.84; 95% CI [0.76, 0.93], p= 0.0009) (Table 3). This finding was driven entirely by a lower incidence of cardiovascular mortality on ENTRESTO.
Table 3: Treatment Effect for the Primary Composite Endpoint, its Components, and All-cause Mortality in PARADIGM-HF *Analyses of the components of the primary composite endpoint were not prospectively planned to be adjusted for multiplicity.
**Includes patients who had heart failure hospitalization prior to death.ENTRESTO
N = 4,187
n (%)Enalapril
N = 4,212
n (%)Hazard Ratio
(95% CI)p-value Primary composite endpoint of cardiovascular death or heart failure hospitalization 914 (21.8) 1,117 (26.5) 0.80 (0.73, 0.87) < 0.0001 Cardiovascular death as first event 377 (9.0) 459 (10.9) Heart failure hospitalization as first event 537 (12.8) 658 (15.6) Number of patients with events: * Cardiovascular death ** 558 (13.3) 693 (16.5) 0.80 (0.71, 0.89) Heart failure hospitalizations 537 (12.8) 658 (15.6) 0.79 (0.71, 0.89) All-cause mortality 711 (17.0) 835 (19.8) 0.84 (0.76, 0.93) 0.0009 The Kaplan-Meier curves presented below (Figure 3) show time to first occurrence of the primary composite endpoint (3A), and time to occurrence of cardiovascular death at any time (3B) and first heart failure hospitalization (3C).
Figure 3: Kaplan-Meier Curves for the Primary Composite Endpoint (A), Cardiovascular Death (B), and Heart Failure Hospitalization (C)
A wide range of demographic characteristics, baseline disease characteristics, and baseline concomitant medications were examined for their influence on outcomes. The results of the primary composite endpoint were consistent across the subgroups examined (Figure 4).
Figure 4: Primary Composite Endpoint (CV Death or HF Hospitalization) - Subgroup Analysis (PARADIGM-HF)
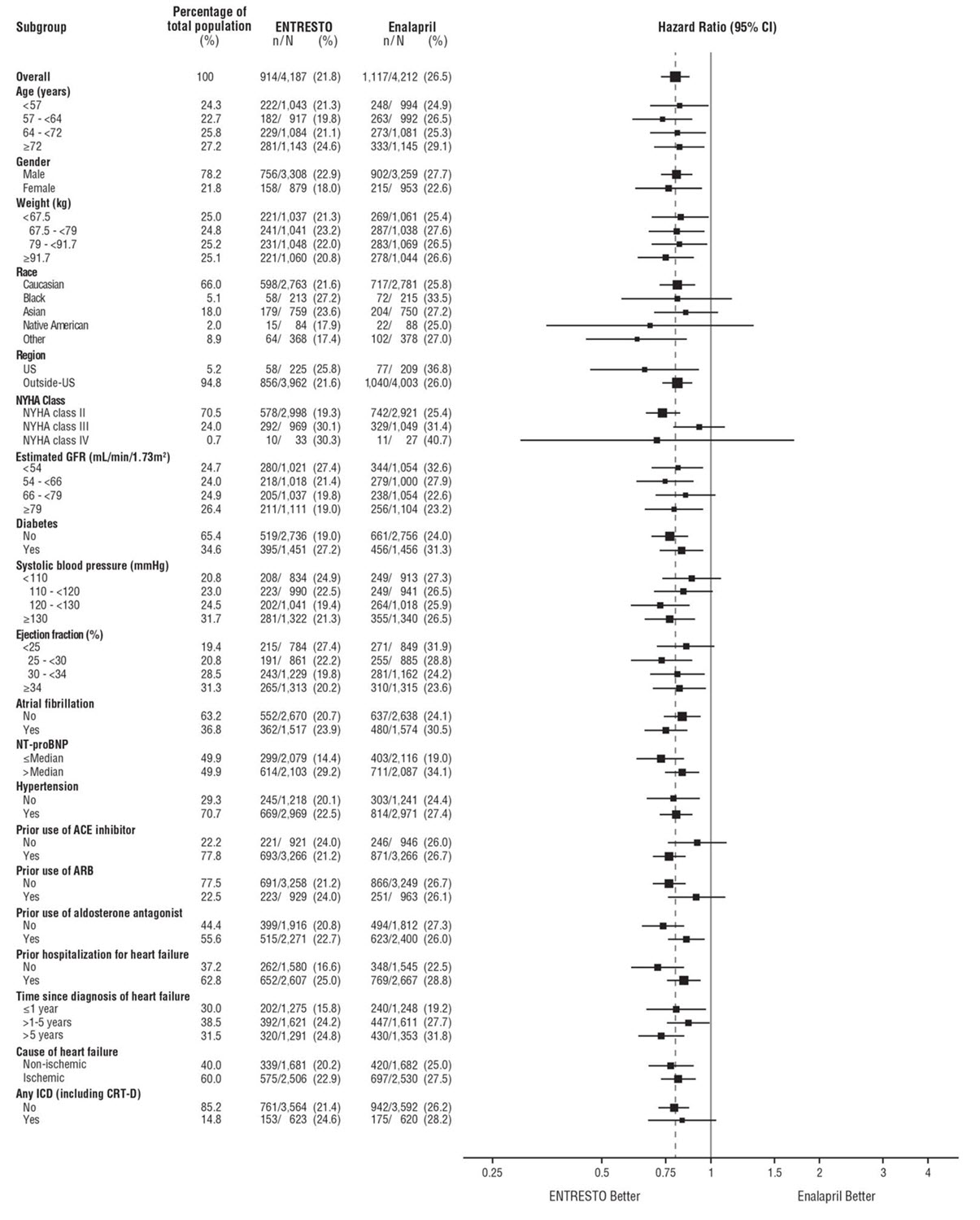
Note: The figure above presents effects in various subgroups, all of which are baseline characteristics. The 95% confidence limits that are shown do not take into account the number of comparisons made, and may not reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted.
PARAGON-HF
PARAGON-HF, was a multicenter, randomized, double-blind trial comparing ENTRESTO and valsartan in 4,796 adult patients with symptomatic heart failure with left ventricular ejection fraction ≥ 45%, and structural heart disease [either left atrial enlargement (LAE) or left ventricular hypertrophy (LVH)]. Patients with a systolic blood pressure of < 110 mmHg and patients with any prior echocardiographic LVEF < 40% at screening were excluded.
The primary objective of PARAGON-HF was to determine whether ENTRESTO reduced the rate of the composite endpoint of total (first and recurrent) heart failure (HF) hospitalizations and cardiovascular (CV) death.
After discontinuing their existing ACE inhibitor or ARB therapy, patients entered sequential single-blind run-in periods during which they received valsartan 80 mg twice-daily, followed by ENTRESTO 100 mg twice-daily. Patients on prior low doses of an ACEi or ARB began the run-in period receiving valsartan 40 mg twice-daily for 1-2 weeks. Patients who successfully completed the sequential run-in periods were randomized to receive either ENTRESTO 200 mg (N = 2,419) twice-daily or valsartan 160 mg (N = 2,403) twice-daily. The median follow-up duration was 35 months and patients were treated for up to 4.7 years.
The population was 81% Caucasian, 13% Asian, and 2% Black; the mean age was 73 years and 52% were female. At randomization, 77% of patients were NYHA Class II, 19% were NYHA Class III, and 0.4% were NYHA Class IV. The median left ventricular ejection fraction was 57%. The underlying cause of heart failure was of ischemic etiology in 36% of patients. Furthermore, 96% had a history of hypertension, 23% had a history of myocardial infarction, 46% had an eGFR < 60 mL/min/1.73 m 2, and 43% had diabetes mellitus. Most patients were taking beta-blockers (80%) and diuretics (95%).
PARAGON-HF demonstrated that ENTRESTO had a numerical reduction in the rate of the composite endpoint of total (first and recurrent) HF hospitalizations and CV death, based on an analysis using a proportional rates model (rate ratio [RR] 0.87; 95% CI [0.75, 1.01], p= 0.06); see Table 4. The treatment effect was primarily driven by the reduction in total HF hospitalizations in patients randomized to ENTRESTO (RR 0.85; 95% CI [0.72, 1.00]).
Table 4: Treatment Effect for the Primary Composite Endpoint and its Components in PARAGON-HF Abbreviations: RR = rate ratio, HR = hazard ratio.
aEvent rate per 100 patient-years.
bIncludes patients who had CV death following HF hospitalization event.ENTRESTO
N = 2,407Valsartan
N = 2,389Effect Size
(95% CI)Efficacy Endpoints n Event Rate a n Event Rate a Composite of total (first and recurrent) HF hospitalizations and CV death 894 12.8 1,009 14.6 RR = 0.87 (0.75, 1.01)
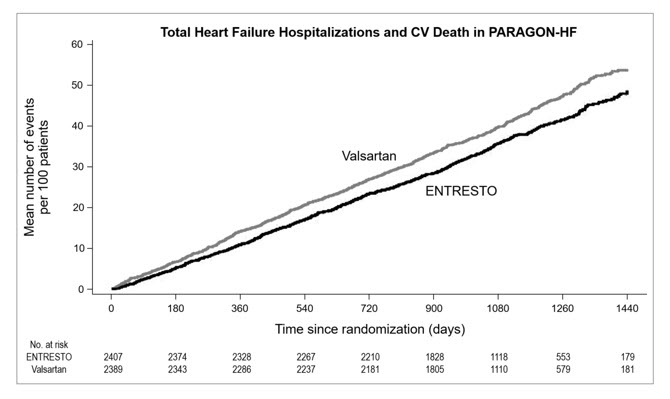
p-value 0.06Total HF Hospitalizations 690 9.9 797 11.6 RR = 0.85 (0.72, 1.00) CV Death b 204 2.9 212 3.1 HR = 0.95 (0.79, 1.16) Figure 5 shows the mean number of composite endpoint events of total HF hospitalizations and CV death over time.
Figure 5: Mean Number of Events Over Time for the Primary Composite Endpoint of Total HF Hospitalizations and CV Death
A wide range of demographic characteristics, baseline disease characteristics, and baseline concomitant medications were examined for their influence on outcomes (Figure 6).
Figure 6: Primary Composite Endpoint of Total HF Hospitalizations and CV Death – Subgroup Analysis (PARAGON-HF)
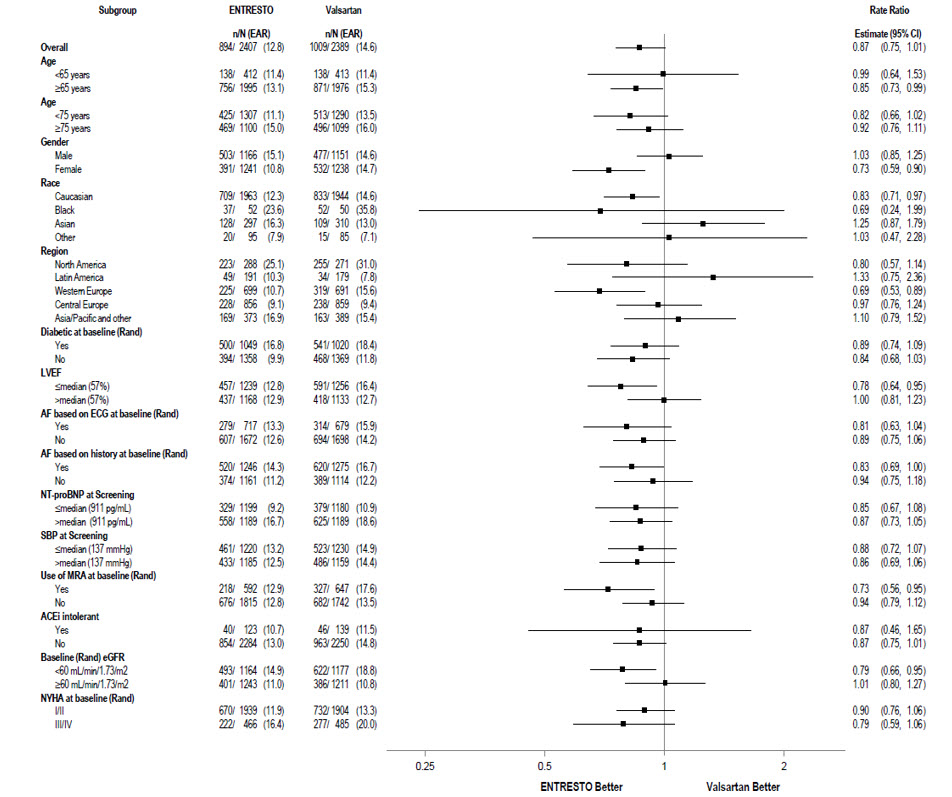
Note: The figure above presents effects in various subgroups, all of which are baseline characteristics. The 95% confidence limits that are shown do not take into account the number of comparisons made, and may not reflect the effect of a particular factor after adjustment for all other factors.
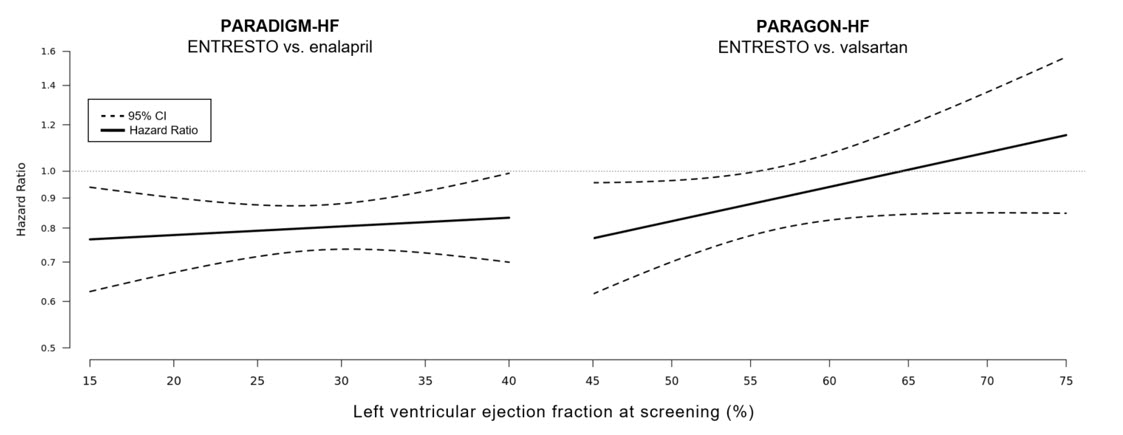
In an analysis of the relationship between LVEF and outcome in PARADIGM-HF and PARAGON-HF, patients with LVEF below normal treated with ENTRESTO experienced greater risk reduction (Figure 7).
Figure 7: Treatment Effect for the Composite Endpoint of Time to First HF Hospitalization or CV Death by LVEF in PARADIGM-HF and PARAGON-HF
14.2 Pediatric Heart Failure
PANORAMA-HF
The efficacy of ENTRESTO was evaluated in a multinational, randomized, double-blind trial comparing ENTRESTO and enalapril based on an analysis in 110 pediatric patients 1 to < 18 years old with heart failure (NYHA/Ross class II-IV) due to systemic left ventricular systolic dysfunction (LVEF ≤ 40%). Patients with systemic right ventricles and single ventricles were excluded from the trial. The target maintenance dose of ENTRESTO in pediatric patients 1 to < 18 years old was 3.1 mg/kg twice daily.
The endpoint was the between-group difference in the change in plasma NT-proBNP from baseline to 12 weeks. The reduction from baseline in NT-proBNP was 44% and 33% in the ENTRESTO and enalapril groups, respectively. While the between-group difference was not statistically significant, the reductions for ENTRESTO and enalapril were similar to or larger than what was seen in adults; these reductions did not appear to be attributable to post-baseline changes in background therapy.
Because ENTRESTO improved outcomes and reduced NT-proBNP in PARADIGM-HF, the effect on NT-proBNP was considered a reasonable basis to infer improved cardiovascular outcomes in pediatric patients.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
ENTRESTO (sacubitril/valsartan) is available as unscored, ovaloid, biconvex, film-coated tablets, containing 24 mg of sacubitril and 26 mg of valsartan; 49 mg of sacubitril and 51 mg of valsartan; and 97 mg of sacubitril and 103 mg of valsartan. All strengths are packaged in bottles as described below.
Tablet Color Debossment NDC # 0078-XXXX-XX Sacubitril/Valsartan “NVR” and Bottle of 60 Bottle of 180 24 mg/26 mg Violet white LZ 0659-20 0659-67 49 mg/51 mg Pale yellow L1 0777-20 0777-67 97 mg/103 mg Light pink L11 0696-20 0696-67 -
17 PATIENT COUNSELING INFORMATION
Advise patients to read the FDA-approved patient labeling (Patient Information).
Pregnancy:Advise female patients of childbearing age about the consequences of exposure to ENTRESTO during pregnancy. Discuss treatment options with women planning to become pregnant. Ask patients to report pregnancies to their physicians as soon as possible [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
Angioedema:Advise patients to discontinue use of their previous ACE inhibitor or ARB. Advise patients to allow a 36 hour wash-out period if switching from or to an ACE inhibitor [see Contraindications (4)and Warnings and Precautions (5.2)].
T2021-08
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: February 2021 Patient Information
ENTRESTO (en-TRESS-toh)
(sacubitril and valsartan) tabletsWhat is the most important information I should know about ENTRESTO?
ENTRESTO can harm or cause death to your unborn baby. Talk to your doctor about other ways to treat heart failure if you plan to become pregnant. If you get pregnant during treatment with ENTRESTO, tell your doctor right away.
What is ENTRESTO?
ENTRESTO is a prescription medicine used to treat:
- adults with long-lasting (chronic) heart failure to help reduce the risk of death and hospitalization. ENTRESTO works better when the heart cannot pump a normal amount of blood to the body.
- certain children 1 year of age and older who have symptomatic heart failure.
It is not known if ENTRESTO is safe and effective in children under 1 year of age.
Do not take ENTRESTO if you:
- are allergic to any of the ingredients in ENTRESTO. See the end of this Patient Information leaflet for a complete list of ingredients in ENTRESTO.
- have had an allergic reaction including swelling of your face, lips, tongue, throat, or trouble breathing while taking a type of medicine called an angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB).
- take an ACE inhibitor medicine. Do not take ENTRESTO for at least 36 hours before or after you take an ACE inhibitor medicine. Talk with your doctor or pharmacist before taking ENTRESTO if you are not sure if you take an ACE inhibitor medicine.
- have diabetes and take a medicine that contains aliskiren.
Before taking ENTRESTO, tell your doctor about all of your medical conditions, including if you:
- have a history of hereditary angioedema
- have kidney or liver problems
- are pregnant or plan to become pregnant. See “What is the most important information I should know about ENTRESTO?”
- are breastfeeding or plan to breastfeed. It is not known if ENTRESTO passes into your breast milk. You and your doctor should decide if you will take ENTRESTO or breastfeed. You should not do both.
Tell your doctor about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using ENTRESTO with certain other medicines may affect each other. Using ENTRESTO with other medicines can cause serious side effects. Especially tell your doctor if you take:
- potassium supplements or a salt substitute
- nonsteroidal anti-inflammatory drugs (NSAIDs)
- lithium
- other medicines for high blood pressure or heart problems such as an ACE inhibitor, ARB, or aliskiren
Keep a list of your medicines to show your doctor and pharmacist when you get a new medicine.
How should I take ENTRESTO?
- Take ENTRESTO exactly as your doctor tells you to take it.
- Take ENTRESTO 2 times each day. Your doctor may change your dose of ENTRESTO during treatment.
- If your child cannot swallow tablets, or if tablets are not available in the prescribed strength, your pharmacist will prepare ENTRESTO as a liquid suspension for your child. If your child switches between taking the tablet and the suspension, your doctor will adjust the dose as needed. Shake the bottle of suspension well before measuring the dose of medicine to give to your child.
- If you miss a dose, take it as soon as you remember. If it is close to your next dose, do not take the missed dose. Take the next dose at your regular time.
- If you take too much ENTRESTO, call your doctor right away.
What are the possible side effects of ENTRESTO?
ENTRESTO may cause serious side effects including:
- See “What is the most important information I should know about ENTRESTO?”
-
Serious allergic reactions causing swelling of your face, lips, tongue, and throat (angioedema) that may cause trouble breathing and death.Get emergency medical help right away if you have symptoms of angioedema or trouble breathing. Do not take ENTRESTO again if you have had angioedema during treatment with ENTRESTO.
People who are Black and take ENTRESTO may have a higher risk of having angioedema than people who are not Black and take ENTRESTO.
People who have had angioedema before taking ENTRESTO may have a higher risk of having angioedema than people who have not had angioedema before taking ENTRESTO. See “Who should not take ENTRESTO?” - Low blood pressure (hypotension).Low blood pressure may be more common if you also take water pills. Call your doctor if you become dizzy or lightheaded, or you develop extreme fatigue.
- Kidney problems.Your doctor will check your kidney function during your treatment with ENTRESTO. If you have changes in your kidney function tests, you may need a lower dose of ENTRESTO or may need to stop taking ENTRESTO for a period of time.
- Increased amount of potassium in your blood (hyperkalemia).Your doctor will check your potassium blood level during your treatment with ENTRESTO.
These are not all the possible side effects of ENTRESTO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ENTRESTO?
- Store ENTRESTO tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Protect ENTRESTO tablets from moisture.
- Store bottles of ENTRESTO oral suspension at room temperature less than 77°F (25°C) for up to 15 days. Do not refrigerate ENTRESTO oral suspension.
Keep ENTRESTO and all medicines out of the reach of children.
General information about the safe and effective use of ENTRESTO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ENTRESTO for a condition for which it was not prescribed. Do not give ENTRESTO to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or doctor for information about ENTRESTO that is written for health professionals.
What are the ingredients in ENTRESTO?
Active ingredients:sacubitril and valsartan
Inactive ingredients:microcrystalline cellulose, low-substituted hydroxypropylcellulose, crospovidone, magnesium stearate (vegetable origin), talc, and colloidal silicon dioxide. Film coat: hypromellose, titanium dioxide (E 171), Macrogol 4000, talc, iron oxide red (E 172). The film-coat for the 24 mg of sacubitril and 26 mg of valsartan tablet and the 97 mg of sacubitril and 103 mg of valsartan tablet also contains iron oxide black (E 172). The film-coat for the 49 mg of sacubitril and 51 mg of valsartan tablet contains iron oxide yellow (E 172).
Prepared ENTRESTO oral suspension also contains Ora-Sweet SF and Ora-Plus.
Distributed by: Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936
© Novartis
ENTRESTO is a registered trademark of Novartis AG
For more information, go to www.ENTRESTO.comor call 1-888-368-7378 (1-888-ENTRESTO).
T2021-09
-
REPACKAGING INFORMATION
Please reference the HOW SUPPLIED section listed above for a description of drug product's characteristics. This drug product has been received by Aphena Pharma Solutions - Tennessee, LLC in a manufacturer or distributor packaged configuration and repackaged in full compliance with all applicable cGMP regulations. The package configurations available from Aphena are listed below:
97mg/103mg
NDC 71610-807-37, Bottles of 3120 Tablets
Store between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:

Cookeville,TN 38506
20240328JK - PRINCIPAL DISPLAY PANEL - 97mg/103mg
-
INGREDIENTS AND APPEARANCE
ENTRESTO
sacubitril and valsartan tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71610-807(NDC:0078-0696) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SACUBITRIL (UNII: 17ERJ0MKGI) (SACUBITRILAT - UNII:SPI5PBF81S) SACUBITRIL 97 mg VALSARTAN (UNII: 80M03YXJ7I) (VALSARTAN - UNII:80M03YXJ7I) VALSARTAN 103 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSPOVIDONE (UNII: 68401960MK) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROXYPROPYL CELLULOSE, LOW SUBSTITUTED (UNII: 2165RE0K14) HYPROMELLOSES (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Product Characteristics Color pink (Light pink) Score no score Shape OVAL Size 15mm Flavor Imprint Code NVR;L11 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71610-807-37 3120 in 1 BOTTLE; Type 0: Not a Combination Product 03/22/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207620 07/07/2015 Labeler - Aphena Pharma Solutions - Tennessee, LLC (128385585) Establishment Name Address ID/FEI Business Operations Aphena Pharma Solutions - Tennessee, LLC 128385585 repack(71610-807)


