Label: TICE BCG- bacillus calmette-guerin powder, for suspension
- NDC Code(s): 0052-0602-01, 0052-0602-02
- Packager: Merck Sharp & Dohme LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
TICE® BCG contains live, attenuated mycobacteria. Because of the potential risk for transmission, prepare, handle, and dispose of TICE® BCG as a biohazard material (see PRECAUTIONS and DOSAGE AND ADMINISTRATION sections).
BCG infections have been reported in health care workers, primarily from exposures resulting from accidental needle sticks or skin lacerations during the preparation of BCG for administration. Nosocomial infections have been reported in patients receiving parenteral drugs that were prepared in areas in which BCG was reconstituted. BCG is capable of dissemination when administered by the intravesical route, and serious infections, including fatal infections, have been reported in patients receiving intravesical BCG (see WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS sections).
-
DESCRIPTION
TICE® BCG for intravesical use, is an attenuated, live culture preparation of the Bacillus of Calmette and Guerin (BCG) strain of Mycobacterium bovis.1 The TICE strain was developed at the University of Illinois from a strain originated at the Pasteur Institute.
The medium in which the BCG organism is grown for preparation of the freeze-dried cake is composed of the following ingredients: glycerin, asparagine, citric acid, potassium phosphate, magnesium sulfate, and iron ammonium citrate. The final preparation prior to freeze drying also contains lactose.
The freeze-dried BCG preparation is delivered in glass vials, each containing 1 to 8 × 108 colony forming units (CFU) of TICE BCG which is equivalent to approximately 50 mg wet weight. Determination of in vitro potency is achieved through colony counts derived from a serial dilution assay. A single dose consists of 1 reconstituted vial (see DOSAGE AND ADMINISTRATION).
For intravesical use the entire vial is reconstituted with sterile saline. TICE BCG is viable upon reconstitution.
No preservatives have been added.
-
CLINICAL PHARMACOLOGY
TICE® BCG induces a granulomatous reaction at the local site of administration. Intravesical TICE BCG has been used as a therapy for, and prophylaxis against, recurrent tumors in patients with carcinoma in situ (CIS) of the urinary bladder, and to prevent recurrence of Stage TaT1 papillary tumors of the bladder at high risk of recurrence. The precise mechanism of action is unknown.
-
CLINICAL STUDIES
Carcinoma in situ (Bladder Cancer)
To evaluate the efficacy of intravesical administration of TICE® BCG in the treatment of carcinoma in situ, patients were identified who had been treated with TICE BCG under 6 different Investigational New Drug (IND) applications in which the most important shared aspect was the use of an induction plus maintenance schedule. Patients received TICE BCG (50 mg; 1 to 8 x 108 CFU) intravesically, once weekly for at least 6 weeks and once monthly thereafter for up to 12 months. A longer maintenance was given in some cases.
The study population consisted of 153 patients, 132 males, 19 females, and 2 unidentified as to gender. Thirty patients lacking baseline documentation of CIS and 4 patients lost to follow-up were not evaluable for treatment response. Therefore, 119 patients were available for efficacy evaluation. The mean age was 69 years (range: 38–97 years).
There were 2 categories of clinical response: (1) Complete Histological Response (CR), defined as complete resolution of carcinoma in situ documented by cystoscopy and cytology, with or without biopsy; and (2) Complete Clinical Response Without Cytology (CRNC), defined as an apparent complete disappearance of tumor upon cystoscopy. The results of a 1987 analysis of the evaluable patients are shown in Table 1.
Table 1: The Response of Patients With CIS Bladder Cancer in 6 IND Studies Entered Evaluable CR CRNC Overall response No. (%) of patients 153 119 (78%) 54 (46%) 36 (30%) 90 (76%) A 1989 update of these data is presented in Table 2. The median duration of follow-up was 47 months.
Table 2: Follow-up Response of Patients With CIS Bladder Cancer in 6 IND Studies 1989 Status of 90 Responders (CR or CRNC) Response 1987/CR
n=541987/CRNC
n=361987 Response
n=90Percent CR 30 15 45 50 CRNC 0 0 0 0 Unrelated deaths 6 6 12 13 Failure 18 15 33 37 There was no significant difference in response rates between patients with or without prior intravesical chemotherapy. The median duration of response, calculated from the Kaplan-Meier curve as median time to recurrence, is estimated at 4 years or greater. The incidence of cystectomy for 90 patients who achieved a complete response (CR or CRNC) was 11%. The median time to cystectomy in patients who achieved a complete response (CR or CRNC) exceeded 74 months.
TaT1 Bladder Cancer
The efficacy of intravesical TICE BCG in preventing the recurrence of a TaT1 bladder cancer after complete transurethral resection of all papillary tumors was evaluated in 2 open-label, randomized phase III clinical trials. Initial diagnosis of patients included in the studies was determined by cystoscopic biopsies. One was conducted by the Southwestern Oncology Group (SWOG) in patients at high risk of recurrence. High risk was defined as 2 occurrences of tumor within 56 weeks, any stage T1 tumor, or 3 or more tumors presenting simultaneously. The second study was conducted at the Nijmegen University Hospital; Nijmegen, The Netherlands. In this study patients were not selected for high risk of recurrence. In both studies treatment was initiated between 1 and 2 weeks after transurethral resection (TUR).
SWOG Trial (study 8795)
In the SWOG trial (study 8795) patients were randomized to TICE BCG or mitomycin C (MMC). Both drugs were given intravesically weekly for 6 weeks, at 8 and 12 weeks, and then monthly for a total treatment duration of 1 year. Cystoscopy and urinary cytology were performed every 3 months for 2 years. Patients with progressive disease or residual or recurrent disease at or after the 6 month follow-up were removed from the study and were classified as treatment failures.
A total of 469 patients was entered into the study: 237 to the TICE BCG arm and 232 to the MMC arm. Twenty-two patients were subsequently found to be ineligible, and 66 patients had concurrent CIS, and were analyzed separately. Four patients were lost to follow-up, leaving 191 evaluable patients in the TICE BCG arm and 186 in the MMC arm. Of the patients, 84% were male and 16% were female. The average age of these patients was 65 years old.
The Kaplan-Meier estimates of 2-year disease-free survival are shown in Table 3. The difference in disease-free survival time between the 2 groups was statistically significant by the log rank test (P=0.03). The 95% confidence interval of the difference in 2-year disease-free survival was 12% ± 10%. No statistically significant differences between the groups were noted in time to tumor progression, tumor invasion, or overall survival.
Table 3: Results of SWOG Study 8795 TICE BCG Arm
N=191MMC Arm
N=186Estimated disease-free survival at 2 years 57% 45% 95% Confidence Interval (CI) (50%, 65%) (38%, 53%) Nijmegen Study
In the Nijmegen study, the efficacy of 3 treatments was compared: TICE substrain BCG, Rijksinstituut voor Volksgezondheid en Milieuhygiene substrain BCG (BCG-RIVM), and MMC.
TICE BCG and BCG-RIVM were given intravesically weekly for 6 weeks. In contrast to the SWOG study, maintenance BCG was not given. Mitomycin C was given intravesically weekly for 4 weeks and then monthly for a total duration of treatment of 6 months. Cystoscopy and urinary cytology were performed every 3 months until recurrence.
A total of 469 patients was enrolled and randomized. Thirty-two patients were not evaluable, 17 were ineligible, 15 were withdrawn before treatment, and 50 had concurrent CIS and were analyzed separately, leaving 387 evaluable patients: 117 in the TICE BCG arm, 134 in the BCG-RIVM arm, and 136 in the MMC arm. Twenty-eight patients (24%) in the TICE BCG arm, 32 patients (24%) in the BCG-RIVM arm, and 24 patients (18%) in the MMC arm had TaG1 tumors. The median duration of follow-up was 22 months (range: 3–54 months).
The Kaplan-Meier estimates of 2-year disease-free survival are shown in Table 4. The differences in disease-free survival among the 3 arms were not statistically significant by the log-rank test (P=0.08).
Table 4: Results of Nijmegen Study TICE BCG Arm
N=117BCG-RIVM Arm
N=134MMC Arm
N=136Estimated disease-free survival at 2 years 53% 62% 64% 95% Confidence Interval (CI) (44%, 64%) (53%, 72%) (55%, 74%) In both the SWOG 8795 study and the Nijmegen study, acute toxicity was more common, and usually more severe, with TICE BCG than with MMC (see ADVERSE REACTIONS).
-
INDICATIONS AND USAGE
TICE® BCG is indicated for:
- the treatment and prophylaxis of carcinoma in situ (CIS) of the urinary bladder
- the prophylaxis of primary or recurrent stage Ta and/or T1 papillary tumors following transurethral resection (TUR)
Limitations of Use:
- TICE BCG is not recommended for stage TaG1 papillary tumors, unless they are judged to be at high risk of tumor recurrence.
- TICE BCG is not indicated for papillary tumors of stages higher than T1.
-
CONTRAINDICATIONS
Immunosuppressed Patients
TICE® BCG should not be used in immunosuppressed patients with congenital or acquired immune deficiencies, whether due to concurrent disease (e.g., AIDS, leukemia, lymphoma) cancer therapy (e.g., cytotoxic drugs, radiation), or immunosuppressive therapy (e.g., corticosteroids).
-
WARNINGS
BCG LIVE (TICE® BCG) is not a vaccine for the prevention of cancer. BCG Vaccine, not BCG LIVE (TICE BCG), should be used for the prevention of tuberculosis. For vaccination use, refer to BCG Vaccine prescribing information.
Handling Precautions
TICE BCG is an infectious agent. Physicians using this product should be familiar with the literature on the prevention and treatment of BCG-related complications, and should be prepared in such emergencies to contact an infectious disease specialist with experience in treating the infectious complications of intravesical BCG. The treatment of the infectious complications of BCG requires long-term, multiple-drug antibiotic therapy. Special culture media are required for mycobacteria, and physicians administering intravesical BCG or those caring for these patients should have these media readily available.
BCG Infection
Instillation of TICE BCG with an actively bleeding mucosa may promote systemic BCG infection. Treatment should be postponed for at least 1 week following transurethral resection, biopsy, traumatic catheterization, or gross hematuria.
Systemic BCG Reaction
Deaths have been reported as a result of systemic BCG infection and sepsis.2,3 Patients should be monitored for the presence of symptoms and signs of toxicity after each intravesical treatment. Febrile episodes with flu-like symptoms lasting more than 72 hours, fever ≥103°F, systemic manifestations increasing in intensity with repeated instillations, or persistent abnormalities of liver function tests suggest systemic BCG infection and may require antituberculous therapy. Local symptoms (prostatitis, epididymitis, orchitis) lasting more than 2 to 3 days may also suggest active infection (see WARNINGS, Management of Serious BCG Complications section).
Laboratory Tests
The use of TICE BCG may cause tuberculin sensitivity. Since this is a valuable aid in the diagnosis of tuberculosis, it is advisable to determine the tuberculin reactivity by PPD skin testing before treatment.
Antimicrobial Therapy
Intravesical instillations of BCG should be postponed during treatment with antibiotics, since antimicrobial therapy may interfere with the effectiveness of TICE BCG (see PRECAUTIONS). TICE BCG should not be used in individuals with concurrent infections.
Bladder Capacity
Small bladder capacity has been associated with increased risk of severe local reactions and should be considered in deciding to use TICE BCG therapy.
Management of Serious BCG Complications
Acute, localized irritative toxicities of TICE BCG may be accompanied by systemic manifestations, consistent with a "flu-like" syndrome. Systemic adverse effects of 1 to 2 days' duration such as malaise, fever, and chills often reflect hypersensitivity reactions. However, symptoms such as fever of ≥38.5°C (101.3°F), or acute localized inflammation such as epididymitis, prostatitis, or orchitis persisting longer than 2 to 3 days suggest active infection, and evaluation for serious infectious complication should be considered.
In patients who develop persistent fever or experience an acute febrile illness consistent with BCG infection, 2 or more antimycobacterial agents should be administered while diagnostic evaluation, including cultures, is conducted. BCG treatment should be discontinued. Negative cultures do not necessarily rule out infection. Physicians using this product should be familiar with the literature on prevention, diagnosis, and treatment of BCG-related complications and, when appropriate, should consult an infectious disease specialist or other physician with experience in the diagnosis and treatment of mycobacterial infections.
TICE BCG is sensitive to the most commonly used antituberculous agents (isoniazid, rifampin, and ethambutol). TICE BCG is not sensitive to pyrazinamide.
-
PRECAUTIONS
General
TICE® BCG contains live mycobacteria and should be prepared and handled using aseptic technique (see DOSAGE AND ADMINISTRATION, Preparation of Agent section). BCG infections have been reported in health care workers preparing BCG for administration. Needle stick injuries should be avoided during the handling and mixing of TICE BCG. Nosocomial infections have been reported in patients receiving parenteral drugs which were prepared in areas in which BCG was prepared.4
BCG is capable of dissemination when administered by intravesical route, and serious reactions, including fatal infections, have been reported in patients receiving intravesical BCG.3 Care should be taken not to traumatize the urinary tract or to introduce contaminants into the urinary system. Seven to 14 days should elapse before TICE BCG is administered following TUR, biopsy, or traumatic catheterization.
TICE BCG should be administered with caution to persons in groups at high risk for HIV infection.
Laboratory Tests
The use of TICE BCG may cause tuberculin sensitivity. It is advisable to determine the tuberculin reactivity of patients receiving TICE BCG by PPD skin testing before treatment is initiated.
Information for Patients
TICE BCG is retained in the bladder for 2 hours and then voided. Patients should void while seated in order to avoid splashing of urine. For the 6 hours after treatment, urine voided should be disinfected for 15 minutes with an equal volume of household bleach before flushing. Patients should be instructed to increase fluid intake in order to "flush" the bladder in the hours following BCG treatment. Patients may experience burning with the first void after treatment.
Patients should be attentive to side effects, such as fever, chills, malaise, flu-like symptoms, or increased fatigue. If the patient experiences severe urinary side effects, such as burning or pain on urination, urgency, frequency of urination, blood in urine, or other symptoms such as joint pain, cough, or skin rash, the physician should be notified.
Drug Interaction
Drug combinations containing immunosuppressants and/or bone marrow depressants and/or radiation interfere with the development of the immune response and should not be used in combination with TICE BCG. Antimicrobial therapy for other infections may interfere with the effectiveness of TICE BCG. There are no data to suggest that the acute, local urinary tract toxicity common with BCG is due to mycobacterial infection, and antituberculosis drugs (e.g., isoniazid) should not be used to prevent or treat the local, irritative toxicities of TICE BCG.
Carcinogenesis, Mutagenesis, Impairment of Fertility
TICE BCG has not been evaluated for its carcinogenic, mutagenic potentials, or impairment of fertility.
Pregnancy
Animal reproduction studies have not been conducted with TICE BCG. It is also not known whether TICE BCG can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. TICE BCG should not be given to a pregnant woman except when clearly needed. Women should be advised not to become pregnant while on therapy.
Nursing Mothers
It is not known whether TICE BCG is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions from TICE BCG in nursing infants, it is advisable to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness of TICE BCG for the treatment of superficial bladder cancer in pediatric patients have not been established.
Geriatric Use
Of the total number of subjects in clinical studies of TICE BCG, the average age was 66 years old. No overall difference in safety or effectiveness was observed between older and younger subjects. Other reported clinical experience has not identified differences in response between elderly and younger patients, but greater sensitivity of some older individuals to BCG cannot be ruled out.
-
ADVERSE REACTIONS
Symptoms of bladder irritability, related to the inflammatory response induced, are reported in approximately 60% of patients receiving TICE® BCG. The symptoms typically begin 4 to 6 hours after instillation and last 24 to 72 hours. The irritative side effects are usually seen following the third instillation, and tend to increase in severity after each administration.
The irritative bladder adverse effects can usually be managed symptomatically with products such as pyridium, propantheline bromide, oxybutynin chloride, and acetaminophen. The mechanism of action of the irritative side effects has not been firmly established, but is most consistent with an immunological mechanism.3 There is no evidence that dose reduction or antituberculous drug therapy can prevent or lessen the irritative toxicity of TICE BCG.
"Flu-like" symptoms (malaise, fever, and chills) which may accompany the localized, irritative toxicities often reflect hypersensitivity reactions which can be treated symptomatically. Antihistamines have also been used.5
Adverse reactions to TICE BCG tend to be progressive in frequency and severity with subsequent instillation. Delay or postponement of subsequent treatment may or may not reduce the severity of a reaction during subsequent instillation.
Although uncommon, serious infectious complications of intravesical BCG have been reported.2,3,6 The most serious infectious complication of BCG is disseminated sepsis with associated mortality. In addition, M. bovis infections have been reported in lung, liver, bone, bone marrow, kidney, regional lymph nodes, and prostate in patients who have received intravesical BCG. Systemic infections may be manifested by pneumonitis, hepatitis, cytopenia, vasculitis, infective aneurysm and/or sepsis after a period of fever and malaise during which symptoms progressively increase. Some male genitourinary tract infections (orchitis/epididymitis) have been resistant to multiple-drug antituberculous therapy and required orchiectomy.
If a patient develops persistent fever or experiences an acute febrile illness consistent with BCG infection, BCG treatment should be discontinued and the patient immediately evaluated and treated for systemic infection (see WARNINGS).
The local and systemic adverse reactions reported in a review of 674 patients with superficial bladder cancer, including 153 patients with carcinoma in situ, are summarized in Table 5.
Table 5: Summary of Adverse Effects Seen in 674 Patients With Superficial Bladder Cancer, Including 153 With Carcinoma in Situ Percent of patients Percent of patients Adverse event N Overall
(Grade ≥3)Adverse event N Overall
(Grade ≥3)Dysuria 401 60% (11%) Arthritis/myalgia 18 3% (<1%) Urinary frequency 272 40% (7%) Headache/dizziness 16 2%(0) Flu-like syndrome 224 33% (9%) Urinary incontinence 16 2% (0) Hematuria 175 26% (7%) Anorexia/weight loss 15 2% (<1%) Fever 134 20% (8%) Urinary debris 15 2% (<1%) Malaise/fatigue 50 7% (0) Allergy 14 2% (<1%) Cystitis 40 6% (2%) Cardiac (unclassified) 13 2% (1%) Urgency 39 6% (1%) Genital inflammation/ Nocturia 30 5% (1%) abscess 12 2% (<1%) Cramps/pain 27 4% (1%) Respiratory (unclassified) 11 2% (<1%) Rigors 22 3% (1%) Urinary tract infection 10 2% (1%) Nausea/vomiting 20 3% (<1%) Abdominal pain 10 2% (1%) The following adverse events were reported in ≤1% of patients: anemia, BCG sepsis, coagulopathy, contracted bladder, diarrhea, epididymitis/prostatitis, hepatic granuloma, hepatitis, leukopenia, neurologic (unclassified), orchitis, pneumonitis, pyuria, rash, thrombocytopenia, urethritis, and urinary obstruction.
In SWOG study 8795, toxicity evaluations were available on a total of 222 TICE BCG-treated patients and 220 MMC-treated patients. Direct bladder toxicity (cramps, dysuria, frequency, urgency, hematuria, hemorrhagic cystitis, or incontinence) was seen more often with TICE BCG with 356 events, compared to 234 events for MMC. Grade ≤2 toxicity was seen significantly more frequently following TICE BCG treatment (P=0.003). No life-threatening toxicity was seen in either arm. Systemic toxicity with TICE BCG was markedly increased compared to that of MMC, with 181 events for TICE BCG compared to 80 for MMC. The frequency of toxicity was increased in all grades, particularly for grades 2 and 3. The most common complaints were malaise, fatigue and lethargy, fever, and abdominal pain. Thirty-two TICE BCG patients were reported to have been treated with isoniazid. Five TICE BCG patients had liver enzyme elevation, including 2 with grade 3 elevations. Eighteen of the 222 (8.1%) TICE BCG patients failed to complete the prescribed protocol compared to 6.2% in the MMC group. Table 6 summarizes the most common adverse reactions reported in this trial.7
Table 6: Most Common Adverse Reactions in SWOG Study 8795* Study arm TICE BCG (N=222) MMC (N=220) Adverse event All Grades Grade ≥3 All Grades Grade ≥3 - *
- The adverse reaction profile of TICE BCG was similar in the Nijmegen study.8
Dysuria 115 (52%) 6 (3%) 77 (35%) 5 (2%) Urgency/frequency 112 (50%) 5 (2%) 63 (29%) 7 (3%) Hematuria 85 (38%) 6 (3%) 56 (25%) 5 (2%) Flu-like symptoms 54 (24%) 1 (<1%) 29 (13%) 0 Fever 37 (17%) 1 (<1%) 7 (3%) 0 Pain (not specified) 37 (17%) 4 (2%) 22 (10%) 1 (<1%) Hemorrhagic cystitis 19 (9%) 3 (1%) 10 (5%) 0 Chills 19 (9%) 0 2 (1%) 0 Bladder cramps 18 (8%) 0 9 (4%) 0 Nausea 16 (7%) 0 12 (5%) 0 Incontinence 8 (4%) 0 3 (1%) 0 Myalgia/arthralgia 7 (3%) 0 0 0 Diaphoresis 7 (3%) 0 1 (<1%) 0 Rash 6 (3%) 1 (<1%) 16 (7%) 2 (1%) -
OVERDOSAGE
Overdosage occurs if more than 1 vial of TICE® BCG is administered per instillation. If overdosage occurs, the patient should be closely monitored for signs of active local or systemic BCG infection. For acute local or systemic reactions suggesting active infection, an infectious disease specialist experienced in BCG complications should be consulted.
-
DOSAGE AND ADMINISTRATION
The dose for the intravesical treatment of carcinoma in situ and for the prophylaxis of recurrent papillary tumors consists of 1 vial of TICE® BCG suspended in 50 mL preservative-free saline.
Do not inject subcutaneously or intravenously.
Preparation of Agent
The preparation of the TICE BCG suspension should be done using aseptic technique. To avoid cross-contamination, parenteral drugs should not be prepared in areas where BCG has been prepared. A separate area for the preparation of the TICE BCG suspension is recommended. All equipment, supplies, and receptacles in contact with TICE BCG should be handled and disposed of as biohazardous. The pharmacist or individual responsible for mixing the agent should wear gloves and take precautions to avoid contact of BCG with broken skin. If preparation cannot be performed in a biocontainment hood, then a mask and gown should be worn to avoid inhalation of BCG organisms and inadvertent exposure to broken skin.
Draw 1 mL of sterile, preservative-free saline (0.9% Sodium Chloride Injection USP) at 4–25°C into a small syringe (e.g., 3 mL) and add to 1 vial of TICE BCG to resuspend. Ensure that the needle is inserted through the center of the rubber stopper of the vial. Gently swirl the vial until a homogenous suspension is obtained. Avoid forceful agitation which may cause clumping of the mycobacteria.
Dilute the cloudy TICE BCG suspension in sterile, preservative-free saline to a final volume of 50 mL. Mix the suspension gently prior to intravesical instillation.
The reconstituted TICE BCG should be kept refrigerated (2–8°C), protected from exposure to direct sunlight, and used within 2 hours. Discard unused portion.
Note: DO NOT filter the contents of the TICE BCG vial. Precautions should be taken to avoid exposing the TICE BCG to direct sunlight. Bacteriostatic solutions must be avoided. In addition, use only sterile, preservative-free saline, 0.9% Sodium Chloride Injection USP as diluent.
Treatment and Schedule
Allow 7 to 14 days to elapse after bladder biopsy before TICE BCG is administered. Patients should not drink fluids for 4 hours before treatment and should empty their bladder prior to TICE BCG administration. The reconstituted TICE BCG is instilled into the bladder by gravity flow via the catheter. After instillation of the TICE BCG suspension is complete, remove the catheter. The TICE BCG is retained in the bladder for 2 hours and then voided. Patients unable to retain the suspension for 2 hours should be allowed to void sooner, if necessary.
While the BCG is retained in the bladder, the patient ideally should be repositioned from left side to right side and also should lie upon the back and the abdomen, changing these positions every 15 minutes to maximize bladder surface exposure to the agent.
A standard treatment schedule consists of 1 intravesical instillation per week for 6 weeks. This schedule may be repeated once if tumor remission has not been achieved and if the clinical circumstances warrant. Thereafter, intravesical TICE BCG administration should continue at approximately monthly intervals for at least 6 to 12 months. There are no data to support the interchangeability of BCG LIVE products.
- HOW SUPPLIED
-
REFERENCES
- DeJager R, Guinan P, Lamm D, Khanna O, Brosman S, DeKernion J, et al. Long-Term Complete Remission in Bladder Carcinoma in Situ with Intravesical TICE Bacillus Calmette Guerin. Urology 1991;38:507–513.
- Rawls WH, Lamm DL, Lowe BA, Crawford ED, Sarosdy MF, Montie JE, Grossman HB, Scardino PT. Fatal Sepsis Following Intravesical Bacillus Calmette-Guerin Administration For Bladder Cancer. J Urol 1990;144:1328–1330.
- Lamm DL, van der Meijden APM, Morales A, Brosman SA, Catalona WJ, Herr HW, et al. Incidence and Treatment of Complications of Bacillus Calmette-Guerin Intravesical Therapy in Superficial Bladder Cancer. J. Urol 1992;147:596–600.
- Stone MM, Vannier AM, Storch SK, Nitta AT, Zhang Y. Brief Report: Meningitis Due to Iatrogenic BCG Infection in Two Immunocompromised Children. NEJM 1995:333:561–563.
- Steg A, Leleu C, Debre B, Gibod-Boccon L, Sicard D. Systemic Bacillus Calmette-Guerin Infection in Patients Treated by Intravesical BCG Therapy for Superficial Bladder Cancer. EORTC Genitourinary Group Monograph 6: BCG in Superficial Bladder Cancer. Edited by F.M. J. Debruyne, L. Denis and A.P.M. van der Meijden. New York: Alan R. Liss Inc., pp. 325–334.
- van der Meijden, APM. Practical Approaches to the Prevention and Treatment of Adverse Reactions to BCG. Eur Urol 1995;27(suppl 1):23–28.
- Lamm DL, Blumenstein BA, Crawford ED, Crissman JD, Lowe BA, Smith JA, Sarosdy MF, Schellhammer PF, Sagalowsky AI, Messing EM, et al. Randomized Intergroup Comparison of Bacillus Calmette-Guerin Immunotherapy and Mitomycin C Chemotherapy Prophylaxis in Superficial Transitional Cell Carcinoma of the Bladder. Urol Oncol 1995;1:119–126.
- Witjes JA, van der Meijden APM, Witjes WPJ, et al. A Randomized Prospective Study Comparing Intravesical Instillations of Mitomycin-C, BCG-Tice, and BCG-RIVM in pTa-pT1 Tumours and Primary Carcinoma In Situ of the Urinary Bladder. Eur J Cancer 1993;29A(12):1672–1676.
-
SPL UNCLASSIFIED SECTION
Manufactured for: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USAManufactured by: Merck Teknika LLC, Durham, NC 27712, USA
U.S. License No. 1747
For patent information: www.msd.com/research/patent
TICE is a registered trademark of The Board of Trustees of the University of Illinois, used under the license of Merck Teknika LLC, Durham, NC, USA.
Copyright © 2021-2022 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.Revised: 8/2022
uspi-v914-pwi-2208r011
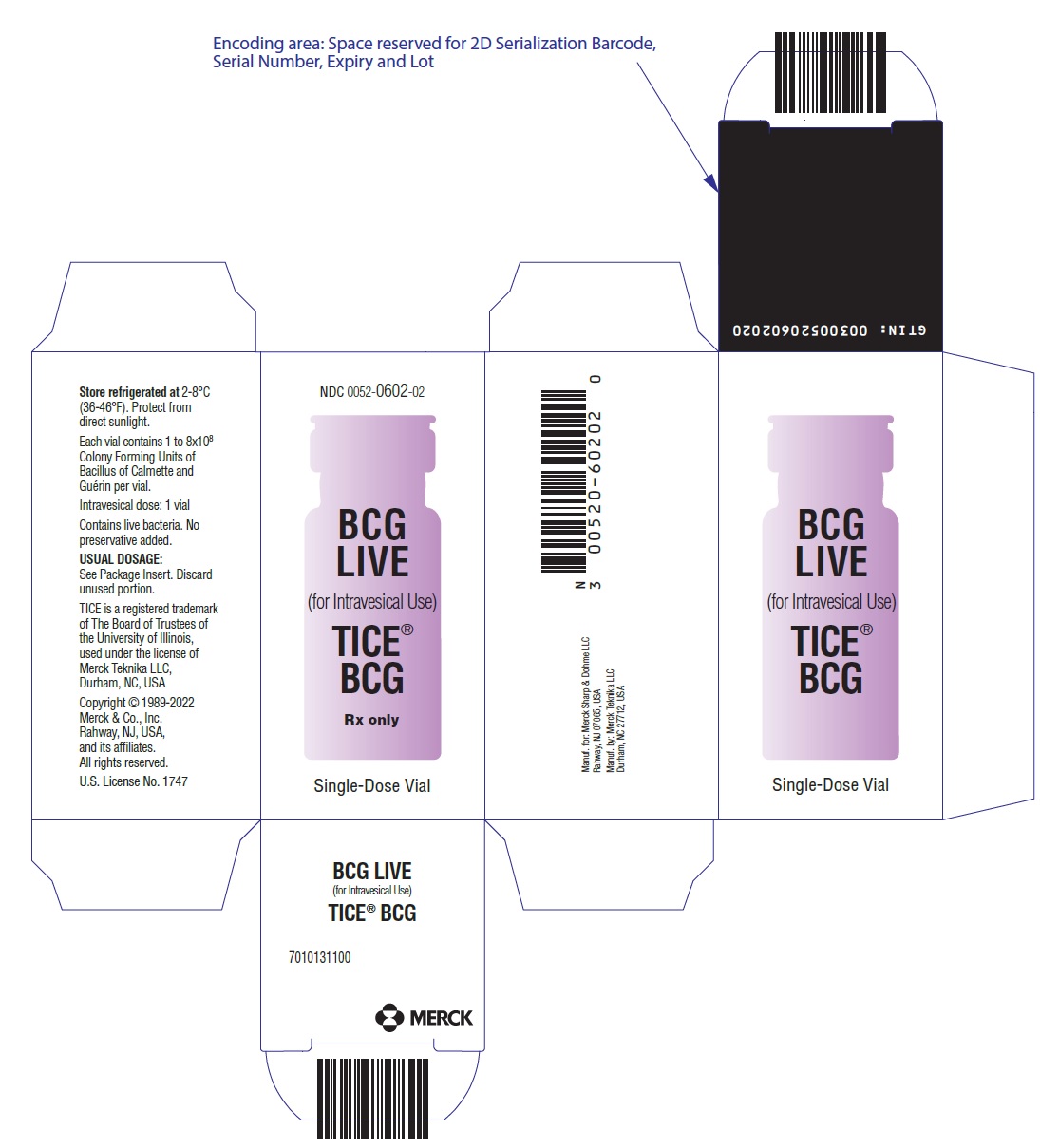
- PRINCIPAL DISPLAY PANEL - 50 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
TICE BCG
bacillus calmette-guerin powder, for suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0052-0602 Route of Administration INTRAVESICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACILLUS CALMETTE-GUERIN SUBSTRAIN TICE LIVE ANTIGEN (UNII: 2XQ558L16Z) (BACILLUS CALMETTE-GUERIN SUBSTRAIN TICE LIVE ANTIGEN - UNII:2XQ558L16Z) BACILLUS CALMETTE-GUERIN SUBSTRAIN TICE LIVE ANTIGEN 50 mg in 50 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) ASPARAGINE (UNII: 5Z33R5TKO7) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM PHOSPHATE, UNSPECIFIED FORM (UNII: B7862WZ632) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) FERRIC AMMONIUM CITRATE (UNII: UVP74NG1C5) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0052-0602-02 1 in 1 CARTON 08/24/1990 1 NDC:0052-0602-01 50 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102821 08/24/1990 Labeler - Merck Sharp & Dohme LLC (118446553)