Label: BIOCHEMISTRY PAIN RELIEF FOOT ACTIVE- benzyl alcohol, lidocaine hydrochloride liquid
- NDC Code(s): 65121-209-31, 65121-209-32
- Packager: Pure Source, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
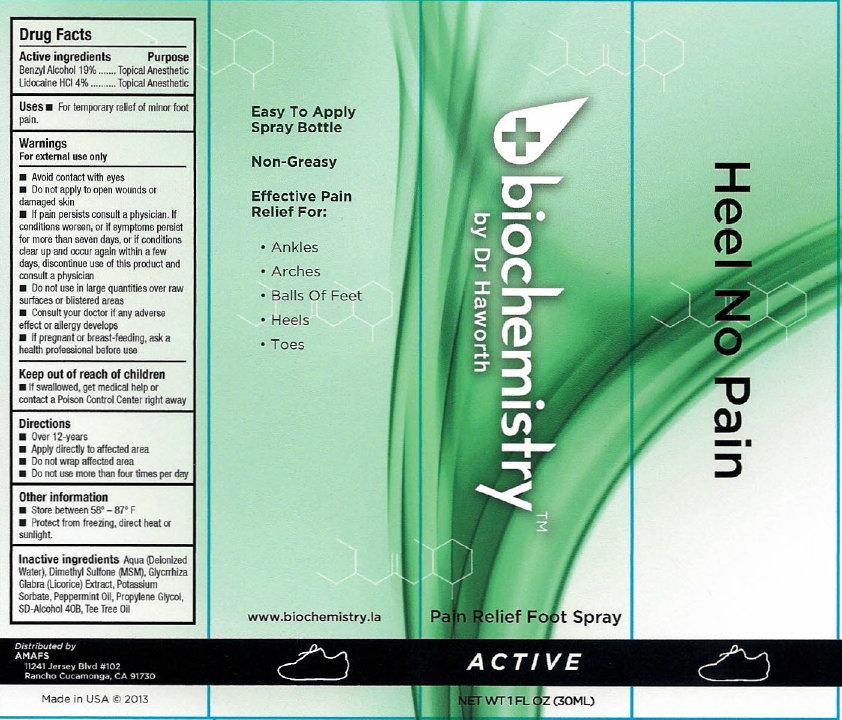
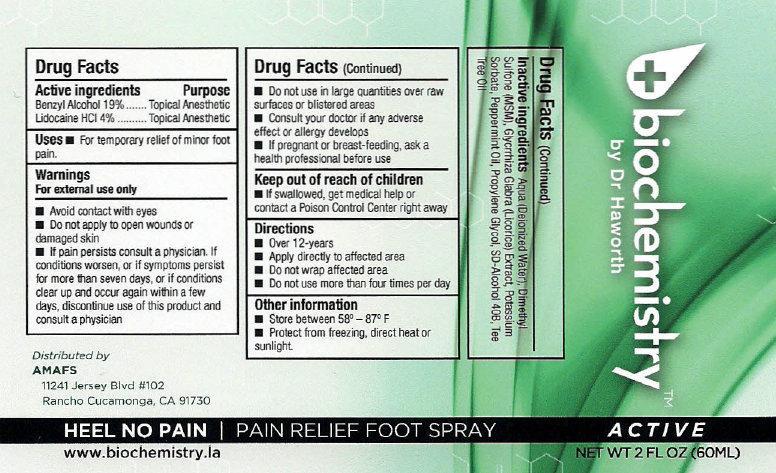
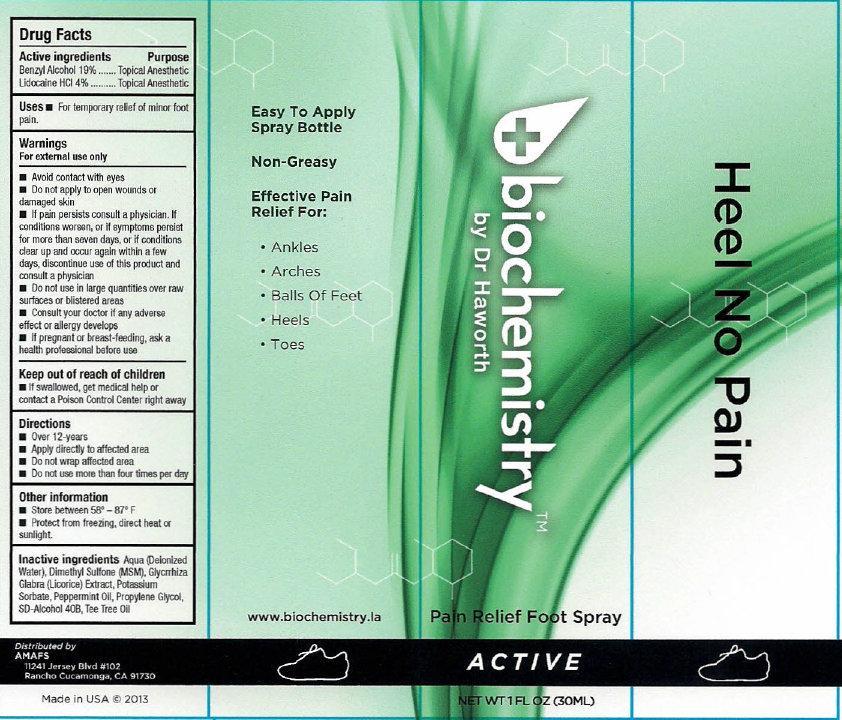
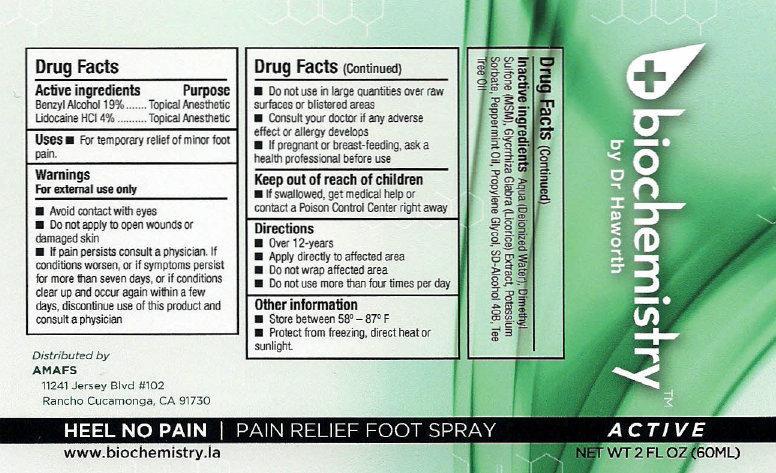
- biochemistry PAIN RELIEF FOOT SPRAY ACTIVE
- Active ingredients
- Uses
-
Warnings
For external use only
Avoid contact with eyes
- Do not apply to open wounds or damaged skin
- If pain persists consult a physician. If conditions worsens, or if symptoms persist for more than seven days, or if conditions clear up and occur again within a few days, discontinue use of this product and consult a physician
- Directions
- Other information
- Inactive ingredients
- biochemistry PAIN RELIEF FOOT SPRAY ACTIVE 30ml (65121-209-31) | biochemistry PAIN RELIEF FOOT SPRAY ACTIVE 60ml (65121-209-32)
-
INGREDIENTS AND APPEARANCE
BIOCHEMISTRY PAIN RELIEF FOOT ACTIVE
benzyl alcohol, lidocaine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65121-209 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZYL ALCOHOL (UNII: LKG8494WBH) (BENZYL ALCOHOL - UNII:LKG8494WBH) BENZYL ALCOHOL 190 mg in 1 mL LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PEPPERMINT OIL (UNII: AV092KU4JH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TEA TREE OIL (UNII: VIF565UC2G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65121-209-31 1 in 1 CARTON 02/09/2017 1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 2 NDC:65121-209-32 1 in 1 CARTON 02/09/2017 2 60 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/06/2014 Labeler - Pure Source, LLC (080354456) Establishment Name Address ID/FEI Business Operations Pure Source, LLC 080354456 manufacture(65121-209)