Label: RAXIBACUMAB injection
- NDC Code(s): 71655-103-01
- Packager: Emergent Manufacturing Operations Baltimore LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 14, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RAXIBACUMAB safely and effectively. See full prescribing information for RAXIBACUMAB.
RAXIBACUMAB injection, for intravenous use
Initial U.S. Approval: 2012WARNING: HYPERSENSITIVITY and ANAPHYLAXIS
See full prescribing information for complete boxed warning
- •
- Hypersensitivity reactions, including anaphylaxis, have been reported during or after the administration of raxibacumab by intravenous infusion (5.1).
- •
- Administer raxibacumab by intravenous infusion in monitored settings where appropriate equipment, medication (including epinephrine) and personnel trained in the management of hypersensitivity, anaphylaxis, and shock are available (2.3, 5.1).
RECENT MAJOR CHANGES
Warnings and Precaution, Hypersensitivity and Anaphylaxis (5.1)
5/2021
5/2021
INDICATIONS AND USAGE
Raxibacumab is indicated for the treatment of adult and pediatric patients with inhalational anthrax due to Bacillus anthracis in combination with appropriate antibacterial drugs, and for prophylaxis of inhalational anthrax when alternative therapies are not available or are not appropriate. (1.1)
Limitations of Use:
- •
- The effectiveness of raxibacumab is based solely on efficacy studies in animal models of inhalational anthrax. (1.2, 14.1)
- •
- There have been no studies of raxibacumab in the pediatric population. Dosing in pediatric patients was derived using an extrapolation approach. (1.2, 8.4)
- •
- Raxibacumab does not cross the blood-brain barrier and does not prevent or treat meningitis. Raxibacumab should be used in combination with appropriate antibacterial drugs. (1.2)
DOSAGE AND ADMINISTRATION
- •
- Premedicate with diphenhydramine. (2.1, 2.2, 5.1)
- •
- Dilute and administer as an intravenous infusion over 2 hours and 15 minutes. (2.3)
- - Adults: 40 mg/kg raxibacumab. (2.1)
- - Pediatrics greater than 40 kg: 40 mg/kg raxibacumab. (2.2)
- - Pediatrics greater than 10 kg to 40 kg: 60 mg/kg raxibacumab. (2.2)
- - Pediatrics 10 kg or less: 80 mg/kg raxibacumab. (2.2)
DOSAGE FORMS AND STRENGTHS
Injection: 1,700 mg/34 mL (50 mg/mL) solution in a single-use vial. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
Hypersensitivity and Anaphylaxis: (Boxed Warning, 2.1, 2.3, 5.1, 6.1)
ADVERSE REACTIONS
Common adverse reactions in healthy adult subjects (≥1.5%) were injection site reaction, erythema and pain, headache, rash, pain in extremity, pruritus, and somnolence. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Emergent BioSolutions at 1-800-768-2304 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Pediatric Use: Safety and effectiveness have not been studied in the pediatric population. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: HYPERSENSITIVITY and ANAPHYLAXIS
1 INDICATIONS AND USAGE
1.1 Inhalational Anthrax
1.2 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Dose and Schedule for Adults
2.2 Dose and Schedule for Pediatric Patients
2.3 Preparation and Administration of Raxibacumab
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity and Anaphylaxis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Ciprofloxacin
7.2 Anthrax Vaccine Adsorbed (AVA)
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology
14 CLINICAL STUDIES
14.1 Treatment of Inhalational Anthrax in Combination with Antibacterial Drug
14.2 Post-Exposure Prophylaxis/Early Treatment of Inhalational Anthrax
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: HYPERSENSITIVITY and ANAPHYLAXIS
- •
- Hypersensitivity reactions, including anaphylaxis, have been reported during or after the administration of raxibacumab by intravenous infusion [see Warnings and Precautions (5.1)].
- •
- Administer raxibacumab by intravenous infusion in monitored settings where appropriate equipment, medication (including epinephrine) and personnel trained in the management of hypersensitivity, anaphylaxis, and shock are available [see Warnings and Precautions (2.3, 5.1)].
-
1 INDICATIONS AND USAGE
1.1 Inhalational Anthrax
Raxibacumab is indicated for the treatment of adult and pediatric patients with inhalational anthrax due to Bacillus anthracis in combination with appropriate antibacterial drugs. Raxibacumab is also indicated for prophylaxis of inhalational anthrax when alternative therapies are not available or are not appropriate.
1.2 Limitations of Use
The effectiveness of raxibacumab is based solely on efficacy studies in animal models of inhalational anthrax. It is not ethical or feasible to conduct controlled clinical trials with intentional exposure of humans to anthrax [see Clinical Studies (14.1)].
Safety and pharmacokinetics (PK) of raxibacumab have been studied in adult healthy volunteers. There have been no trials of safety or PK of raxibacumab in the pediatric population. An extrapolation approach was used to derive dosing regimens that are predicted to provide pediatric patients with exposure comparable to the observed exposure in adults [see Use in Specific Populations (8.4)].
Raxibacumab binds to the protective antigen (PA) of B. anthracis; it does not have direct antibacterial activity. Raxibacumab does not cross the blood-brain barrier and does not prevent or treat meningitis. Raxibacumab should be used in combination with appropriate antibacterial drugs.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dose and Schedule for Adults
Administer raxibacumab as a single dose of 40 mg/kg intravenously over 2 hours and 15 minutes after dilution in 0.9% Sodium Chloride Injection, USP (normal saline) to a final volume of 250 mL. Administer 25 to 50 mg diphenhydramine within 1 hour prior to the raxibacumab infusion to reduce the risk of occurrence and/or the severity of an infusion reaction. Diphenhydramine route of administration (oral or intravenous) should be based on the temporal proximity to the start of the raxibacumab infusion [see Warnings and Precautions (5.1), Adverse Reactions (6.1)].
2.2 Dose and Schedule for Pediatric Patients
The recommended dose for pediatric patients is based on weight as shown in Table 1.
Table 1. Recommended Pediatric Dose Pediatric Body Weight Pediatric Dose Greater than 40 kg
40 mg/kg
Greater than 10 kg to 40 kg
60 mg/kg
10 kg or less
80 mg/kg
Premedicate with diphenhydramine within 1 hour prior to the raxibacumab infusion to reduce the risk of occurrence and/or the severity of an infusion reaction. Diphenhydramine route of administration (oral or intravenous) should be based on the temporal proximity to the start of the raxibacumab infusion. Infuse raxibacumab over 2 hours and 15 minutes. No pediatric patients were studied during the development of raxibacumab. The dosing recommendations in Table 1 are derived from simulations designed to match the observed adult exposure to raxibacumab at a 40 mg/kg dose [see Use in Specific Populations (8.4)].
2.3 Preparation and Administration of Raxibacumab
The recommended dose of raxibacumab is weight-based, given as an intravenous infusion after dilution in a compatible solution to a final volume of 250 mL in adults or to a volume indicated based on the child’s weight (Table 2). Dilute raxibacumab using one of the following compatible solutions:
- •
- 0.9% Sodium Chloride Injection, USP
- •
- 0.45% Sodium Chloride Injection, USP
Keep vials in their cartons prior to preparation of an infusion solution to protect raxibacumab from light. Raxibacumab vials contain no preservative.
Table 2. Raxibacumab Dose, Diluents, Infusion Volumes, and Rates by Body Weight a For patients requiring maximal fluid restriction, dilution factors can be adjusted at the discretion of the physician to a maximal concentration of 32 mg/mL. Preparation
Administration
Body Weight
Dose
Total
Infusion
Volumea
Type of Diluent
Infusion Rate
Infusion Rate
First 20 Minutes
Remaining Infusion
Adults
40 mg/kg
250 mL
0.9% Sodium Chloride Injection
15 mL/hour
125 mL/hour
Pediatric Patients (Younger than 18 Years)
Less than 1 kg
80 mg/kg
7 mL
0.45% Sodium Chloride Injection
or
0.9% Sodium Chloride Injection
0.5 mL/hour
3.5 mL/hour
1 kg to less than 2 kg
15 mL
1 mL/hour
7 mL/hour
2 kg to less than 3 kg
20 mL
1.2 mL/hour
10 mL/hour
3 kg to less than 5 kg
25 mL
1.5 mL/hour
12 mL/hour
5 kg to 10 kg
40 mL
3 mL/hour
20 mL/hour
Greater than 10 kg to less than 15 kg
60 mg/kg
50 mL
0.9% Sodium Chloride Injection
6 mL/hour
25 mL/hour
15 kg to less than 30 kg
100 mL
6 mL/hour
50 mL/hour
30 kg to 40 kg
125 mL
15 mL/hour
62.5 mL/hour
Greater than 40 kg
40 mg/kg
150 mL
15 mL/hour
75 mL/hour
Preparation
Follow the steps below to prepare the raxibacumab intravenous infusion solution.
- 1.
- Calculate the milligrams of raxibacumab injection by multiplying the recommended mg/kg dose in Table 2 by patient weight in kilograms.
- 2.
- Calculate the required volume in milliliters of raxibacumab injection needed for the dose by dividing the calculated dose in milligrams (Step 1) by the concentration, 50 mg/mL. Each single-use vial allows delivery of 34 mL raxibacumab.
Based on the total infusion volume selected in Table 2, prepare either a syringe or infusion bag as appropriate following the steps below.
Syringe Preparation
- 3.
- Select an appropriate size syringe for the total volume of infusion to be administered, as described in Table 2.
- 4.
- Using the selected syringe, withdraw the volume of raxibacumab as calculated in Step 2.
- 5.
- Withdraw an appropriate amount of compatible solution to prepare a total volume infusion syringe as specified in Table 2.
- 6.
- Gently mix the solution. Do not shake.
- 7.
- Discard any unused portion remaining in the raxibacumab vial(s).
- 8.
- The prepared solution is stable for 8 hours stored at room temperature.
Infusion Bag Preparation
- 3.
- Select appropriate size bag of compatible solution (see compatible solutions listed in Table 2); withdraw a volume of solution from the bag equal to the calculated volume in milliliters of raxibacumab in Step 2 above. Discard the solution that was withdrawn from the bag.
- 4.
- Withdraw the required volume of raxibacumab injection from the raxibacumab vial(s).
- 5.
- Transfer the required volume of raxibacumab injection to the selected infusion bag (Step 3). Gently invert the bag to mix the solution. Do not shake.
- 6.
- Discard any unused portion remaining in the raxibacumab vial(s).
- 7.
- The prepared solution is stable for 8 hours stored at room temperature.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard the solution if particulate matter is present or color is abnormal [see Description (11)].
Administration
- •
- Administer raxibacumab in monitored settings appropriately equipped to manage hypersensitivity, anaphylaxis, and shock [see Warnings and Precautions (5.1)].
- •
- Administer the infusion solution as described in Table 2. The rate of infusion may be slowed or interrupted if the patient develops any signs of adverse reactions, including infusion-associated symptoms.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity and Anaphylaxis
Hypersensitivity reactions including rash, urticaria, pruritus, chills, chest and throat tightness, lip and throat swelling, and hypotension were reported in 27 (4.5%) of 606 healthy subjects during or after the administration of raxibacumab in clinical trials. Two subjects experienced anaphylaxis during the raxibacumab infusion [see Adverse Reactions (6.1)].
Some subjects with hypersensitivity or anaphylaxis required interruption or discontinuation of the raxibacumab infusion as well as additional appropriate treatment that included steroids, diphenhydramine, H2 blockers, and/or intravenous fluids [see Adverse Reactions (6.1)].
Due to the risk of anaphylaxis, administer raxibacumab injection in monitored settings where appropriate equipment, medication (including epinephrine) and personnel trained to manage hypersensitivity, anaphylaxis, and shock are available. Monitor patients closely during the infusion and for a period of time after administration. If hypersensitivity reactions or anaphylaxis occur, interrupt or stop the raxibacumab infusion immediately and treat appropriately.
Premedicate with diphenhydramine within 1 hour prior to administering raxibacumab to reduce the risk of occurrence and/or severity of a hypersensitivity reaction [see Dosage and Administration (2.1), Adverse Reactions (6.1)]. Diphenhydramine premedication does not prevent anaphylaxis and may mask or delay the onset of symptoms of hypersensitivity.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice. The safety of raxibacumab has been studied only in healthy volunteers. It has not been studied in patients with inhalational anthrax.
In the three pre-licensure clinical trials (Trials 1, 2, and 3), the safety of raxibacumab was evaluated in 326 healthy subjects treated with a dose of 40 mg/kg in 3 clinical trials: a drug interaction trial with ciprofloxacin (Trial 1), a repeat-dose trial of 20 subjects with the second raxibacumab dose administered ≥4 months after the first dose (Trial 2), and a placebo-controlled trial evaluating single doses with a subset of subjects receiving 2 raxibacumab doses 14 days apart (Trial 3). Raxibacumab was administered to 86 healthy subjects in Trial 1. In Trial 3, 240 healthy subjects received raxibacumab (217 received 1 dose and 23 received 2 doses) and 80 subjects received placebo.
The overall safety of raxibacumab was evaluated as an integrated summary of these 3 clinical trials. Of 326 raxibacumab subjects, 283 received single doses, 23 received 2 doses 14 days apart, and 20 received 2 doses more than 4 months apart. The subjects were aged 18 to 88 years, 53% female, 74% white, 17% black/African American, 6% Asian, and 15% Hispanic.
Trial 4 was a post-marketing trial in healthy subjects designed to evaluate the effect of a single 40 mg/kg infusion of raxibacumab on the immunogenicity of a concurrently administered 3-dose subcutaneous (SC) regimen of Anthrax Vaccine Adsorbed (AVA) [N=286] compared with an AVA-alone regimen [N=286]. In the combination arm, the first dose of AVA was administered immediately following the raxibacumab infusion (Day 1), while the second and third doses were administered on Days 15 and 29. The mean age of subjects was 36 years in both arms, 52% of subjects in the AVA arm and 50% in the AVA + raxibacumab arm were female, 75% and 77% in the two arms, respectively, were white, while 21% and 20%, respectively, were African American.
Adverse Reactions Leading to Discontinuation or Interruption of Raxibacumab Infusion
Four subjects (1.2%) in Trials 1, 2 and 3 had their infusion of raxibacumab discontinued for hypersensitivity and anaphylaxis: 2 subjects (neither of whom received diphenhydramine premedication) discontinued due to urticaria (mild), and 1 subject each discontinued for clonus (mild) and dyspnea (moderate).
In Trial 4, six (2.1%) subjects required discontinuation of the raxibacumab infusion and 3 (1.0%) subjects required infusion interruption due to hypersensitivity or anaphylaxis [see Warnings and Precautions (5.1)].
Most Frequently Reported Adverse Reactions
Trials 1, 2, and -3
In Trials 1, 2, and 3, the most frequently reported adverse reactions were rash, pain in extremity, pruritus, and somnolence (Table 3).
Table 3. Adverse Reactions Reported in ≥1.5% of Healthy Adult Subjects Exposed to Raxibacumab 40 mg/kg Intravenously in Trials 1, 2, and 3 Adverse Reaction
Placebo
N = 80 (%)
Single-Dose Raxibacumab
N = 283 (%)
Double-Dose Raxibacumab
≥4 Months Apart
N = 20 (%)
Double-Dose Raxibacumab
2 Weeks Apart
N = 23 (%)
Total Raxibacumab Subjects
N = 326 (%)
Rash/Rash erythematous/Rash papular
1 (1.3)
9 (3.2)
0
0
9 (2.8)
Pain in extremity
1 (1.3)
7 (2.5)
0
0
7 (2.1)
Pruritus
0
7 (2.5)
0
0
7 (2.1)
Somnolence
0
4 (1.4)
0
1 (4.3)
5 (1.5)
Rashes
For all subjects exposed to raxibacumab in Trials 1, 2, and 3, the rate of rash was 2.8% (9/326) compared with 1.3% (1/80) of placebo subjects. Mild to moderate infusion-related rashes were reported in 22.2% (6/27) of subjects who did not receive diphenhydramine premedication compared with 3.3% (2/61) of subjects who were premedicated with diphenhydramine in the ciprofloxacin/raxibacumab combination trial (Trial 1). In the placebo-controlled raxibacumab trial where all subjects received diphenhydramine (Trial 3), the rate of rash was 2.5% in both placebo- and raxibacumab-treated subjects.
Trial 4
Eleven (3.8%) of 286 subjects who received raxibacumab, all of whom received diphenhydramine premedication, experienced hypersensitivity manifesting as urticaria, pruritus, lip and throat swelling, chest and throat tightness, hypotension, and diffuse erythema; 2 (0.7%) of these subjects had anaphylaxis. Raxibacumab infusion was discontinued or interrupted in 9/11 subjects. Hypersensitivity reactions and anaphylaxis occurred during administration of raxibacumab prior to administration of the first dose of AVA [see Warnings and Precautions (5.1)].
Common adverse reactions, other than hypersensitivity, occurring at a frequency of ≥1.5% in raxibacumab-exposed subjects are presented in Table 4.
Table 4. Adverse Reactions Other than Hypersensitivity Reported in ≥1.5% of Healthy Adult Subjects Exposed to Raxibacumab 40 mg/kg Intravenously in Trial 4 Adverse Reaction
AVA + Raxibacumab Subjects
N = 286 (%)
AVA Subjects
N = 286 (%)
Injection site reaction
18 (6.3)
18 (6.3)
Injection site erythema
13 (4.5)
11 (3.8)
Headache
9 (3.1)
6 (2.1)
Injection site pain
8 (2.8)
6 (2.1)
Less Common Adverse Reactions
Clinically significant adverse reactions that were reported in <1.5% of subjects exposed to raxibacumab and at rates higher than in placebo subjects are listed below:
- •
- Blood and Lymphatic System Disorders: Anemia, leukopenia, lymphadenopathy.
- •
- Cardiac Disorders: Palpitations.
- •
- Ear and Labyrinth Disorders: Vertigo.
- •
- General Disorders and Administration Site Conditions: Fatigue, infusion site pain, peripheral edema.
- •
- Investigations: Blood amylase increased, blood creatine phosphokinase increased, prothrombin time prolonged.
- •
- Musculoskeletal and Connective Tissue Disorders: Back pain, muscle spasms.
- •
- Nervous System Disorders: Syncope.
- •
- Psychiatric Disorders: Insomnia.
- •
- Vascular Disorders: Flushing, hypertension.
Immunogenicity
The development of anti-raxibacumab antibodies was evaluated in all subjects receiving single and double doses of raxibacumab in Trials 1, 2, and 3. Immunogenic responses against raxibacumab were not detected in any raxibacumab-treated human subjects following single or repeat doses of raxibacumab.
The incidence of antibody formation is highly dependent on the sensitivity and specificity of the immunogenicity assay. Additionally, the observed incidence of any antibody positivity in an assay is highly dependent on several factors, including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to raxibacumab with the incidence of antibodies to other products may be misleading.
-
7 DRUG INTERACTIONS
7.1 Ciprofloxacin
Co-administration of 40 mg/kg raxibacumab intravenously with intravenous or oral ciprofloxacin in human subjects did not alter the PK of either ciprofloxacin or raxibacumab [see Clinical Pharmacology (12.3)].
7.2 Anthrax Vaccine Adsorbed (AVA)
Co-administration of 40 mg/kg raxibacumab intravenously (Day 1) with a SC AVA regimen (Days 1, 15, and 29) did not affect the immunogenicity of AVA [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data on the use of raxibacumab in pregnant women to inform on drug-associated risk. In pregnant rabbits, intravenous administration of raxibacumab was not associated with teratogenicity or other adverse developmental outcomes at 3 times the human maximum plasma concentrations at the maximum recommended adult dose (see Data).
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background rate of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk: Limited data in the form of case reports of anthrax infection in pregnant women indicate that maternal infection is associated with a high risk of maternal, fetal, and neonatal deaths, particularly in the absence of treatment.
Data
Animal Data: A study was conducted in pregnant, healthy New Zealand White rabbits administered intravenous raxibacumab at dose levels of 40 or 120 mg/kg on Gestation Days 7 and 14. No teratogenicity or other adverse developmental outcomes were observed in pregnant rabbits at 3 times the human maximum plasma concentrations at the maximum recommended adult dose of 40 mg/kg. Maternal toxicity was observed at both doses (reduced body weight gain late in gestation, but no difference in mean total weight gain).
8.2 Lactation
Risk Summary
There is no information available on the presence of raxibacumab in human milk, the effects on the breastfed child, or the effects on milk production. Maternal IgG is known to be present in human milk.
Therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for raxibacumab and any potential adverse effects on the breastfed child from raxibacumab or from the underlying maternal condition.
8.4 Pediatric Use
As in adults, the effectiveness of raxibacumab in pediatric patients is based solely on efficacy studies in animal models of inhalational anthrax. As exposure of healthy children to raxibacumab is not ethical, an extrapolation approach was used to derive dosing regimens that are predicted to provide pediatric patients with exposure comparable to the observed exposure in adults receiving 40 mg/kg. The dose for pediatric patients is based on weight [see Dosage and Administration (2.2)].
Safety or PK of raxibacumab have not been studied in the pediatric population.
8.5 Geriatric Use
Clinical trials of raxibacumab did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently from younger subjects. Of the total number of subjects in clinical trials of raxibacumab, 6.4% (21/326) were 65 years and older, while 1.5% (5/326) were 75 years and older. However, no alteration of dosing is needed for patients aged 65 years and older [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Raxibacumab is a human IgG1λ monoclonal antibody that binds the PA component of B. anthracis toxin. Raxibacumab has a molecular weight of approximately 146 kilodaltons. Raxibacumab is produced by recombinant DNA technology in a murine cell expression system.
Raxibacumab is supplied as a sterile, preservative-free, clear to opalescent, colorless to pale yellow liquid formulation in single-use vials for intravenous infusion. Each vial contains 1,700 mg/34 mL (50 mg/mL) raxibacumab in citric acid (0.13 mg/mL), glycine (18 mg/mL), polysorbate 80 [0.2 mg/mL (w/v)], sodium citrate (2.8 mg/mL), sucrose (10 mg/mL), and Water for Injection, with a pH of 6.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Raxibacumab is a monoclonal antibody that binds the protective antigen of B. anthracis [see Microbiology (12.4)].
12.3 Pharmacokinetics
The PK of raxibacumab is linear over the dose range of 1 to 40 mg/kg following single intravenous dosing in humans; raxibacumab was not tested at doses higher than 40 mg/kg in humans. Following single intravenous administration of raxibacumab 40 mg/kg in healthy male and female human subjects, the mean Cmax and AUCinf were 1,020.3 ± 140.6 mcg/mL and 15,845.8 ± 4,333.5 mcg•day/mL, respectively. Mean raxibacumab steady-state volume of distribution was greater than plasma volume, suggesting some tissue distribution. Clearance values were much smaller than the glomerular filtration rate indicating that there is virtually no renal clearance of raxibacumab.
Because the effectiveness of raxibacumab cannot be tested in humans, a comparison of raxibacumab exposures achieved in healthy human subjects to those observed in animal models of inhalational anthrax in therapeutic efficacy studies is necessary to support the dosage regimen of 40 mg/kg intravenously as a single dose for the treatment of inhalational anthrax in humans. Humans achieve similar or greater systemic exposure (Cmax and AUCinf) to raxibacumab following a single 40 mg/kg intravenous dose compared with New Zealand White rabbits and cynomolgus macaques receiving the same dosage regimen.
Effects of Gender, Age, and Race
Raxibacumab PK was evaluated via a population PK analysis using serum samples from 322 healthy subjects who received a single 40 mg/kg intravenous dose across 3 clinical trials. Based on this analysis, gender (female versus male), race (non-white versus white), or age (elderly versus young) had no meaningful effects on the PK parameters for raxibacumab.
Raxibacumab PK has not been evaluated in children [see Dosage and Administration (2.2), Use in Specific Populations (8.4)].
Repeat Dosing
Although raxibacumab is intended for single-dose administration, the PK of raxibacumab following a second administration of 40 mg/kg given intravenously 14 days after the first 40 mg/kg intravenous dose was assessed in 23 healthy subjects (Trial 3). The mean raxibacumab concentration at 28 days after the second dose was approximately twice the mean raxibacumab concentration at 14 days following the first dose. In the human trial assessing the immunogenicity of raxibacumab (Trial 2), 20 healthy subjects who had initially received a single dose of raxibacumab 40 mg/kg intravenously received a second 40 mg/kg intravenous dose at ≥4 months following their first dose. No statistically significant differences in mean estimates of AUCinf, CL, or half-life of raxibacumab between the 2 doses administered ≥4 months apart were observed. The mean Cmax following the second dose was 15% lower than the Cmax following the first dose.
Ciprofloxacin Interaction Trial
In an open-label trial evaluating the effect of raxibacumab on ciprofloxacin PK in healthy adult male and female subjects (Trial 1), the administration of 40 mg/kg raxibacumab intravenously following ciprofloxacin intravenous infusion or ciprofloxacin oral tablet ingestion did not alter the PK of ciprofloxacin administered orally and/or intravenously. Likewise, ciprofloxacin did not alter the PK of raxibacumab [see Drug Interactions (7.1)].
Co-Administration with AVA Trial
The effect of a single 40 mg/kg infusion of raxibacumab on the immunogenicity of a three-dose regimen of AVA administered (SC) was assessed in an open-label randomized trial in healthy adult male and female subjects (Trial 4). The trial included two treatment arms. In the combination arm, the first dose of AVA was administered immediately following the raxibacumab infusion (Day 1), while the second and third doses were administered on Days 15 and 29. In the AVA-alone arm, these SC AVA doses were administered on Days 1, 15 and 29. Overall immune response to AVA in the combination arm was found to be similar to the AVA-alone arm.
12.4 Microbiology
Mechanism of Action
Raxibacumab is a monoclonal antibody that binds free PA with an affinity equilibrium dissociation constant (Kd) of 2.78 ± 0.9 nM. Raxibacumab inhibits the binding of PA to its cellular receptors, preventing the intracellular entry of the anthrax lethal factor and edema factor, the enzymatic toxin components responsible for the pathogenic effects of anthrax toxin.
Activity In Vitro and In Vivo
Raxibacumab binds in vitro to PA from the Ames, Vollum, and Sterne strains of B. anthracis. Raxibacumab binds to an epitope on PA that is conserved across reported strains of B. anthracis.
In vivo studies in rats suggest that raxibacumab neutralizes the toxicity due to lethal toxin, as animals slowly infused with lethal toxin (a combination of PA + lethal factor) survived 7 days following administration. The median time to death in control rats was 16 hours. Similar observations were noted in animal efficacy studies in rabbits and monkeys challenged with B. anthracis spores by the inhalational route. PA was detected in animals following exposure to B. anthracis spores. PA levels rose and then fell to undetectable levels in animals that responded to treatment and survived, whereas levels continued to rise in animals that failed treatment and died or were euthanized because of poor clinical condition [see Clinical Studies (14.1)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity, genotoxicity, and fertility studies have not been conducted with raxibacumab.
13.2 Animal Toxicology
Healthy cynomolgus macaques administered 3 intravenous doses or 3 SC doses of 40 mg/kg raxibacumab once every 12 days, or a single intramuscular dose (40 mg/kg) of raxibacumab, showed no adverse effects, including no effects up to 120 days post-dosing.
Studies with raxibacumab in rabbit, cynomolgus macaque, and human donor tissues showed no cross reactivity with brain.
Anthrax-infected rabbits and monkeys administered an intravenous injection of raxibacumab (40 mg/kg) at time of PA toxemia reproducibly showed greater severity of central nervous system (CNS) lesions (bacteria, inflammation, hemorrhage, and necrosis) in non-surviving animals compared with dead placebo-control animals, with no difference in mean time to death from spore challenge. The raxibacumab monoclonal antibody appears unable to penetrate the CNS until compromise of the blood-brain barrier (BBB) during the later stages of anthrax infection. The most severe brain lesions in rabbits were associated with bacteria and raxibacumab tissue binding in a similar pattern as endogenous IgG antibody that leaked across the compromised BBB. No dose/exposure-response relationship for brain histopathology was identified. Surviving rabbits and monkeys at the end of the 28-day studies showed no microscopic evidence of CNS lesions. CNS toxicity was not observed in healthy monkeys administered raxibacumab (40 mg/kg) or in GLP combination treatment studies with antibacterials in rabbits (levofloxacin) or in monkeys (ciprofloxacin) at any time.
-
14 CLINICAL STUDIES
Because it is not feasible or ethical to conduct controlled clinical trials in humans with inhalational anthrax, the effectiveness of raxibacumab for therapeutic treatment of inhalational anthrax is based on efficacy studies in rabbits and monkeys. Raxibacumab effectiveness has not been studied in humans. Because the animal efficacy studies are conducted under widely varying conditions, the survival rates observed in the animal studies cannot be directly compared between studies and may not reflect the rates observed in clinical practice.
The efficacy of raxibacumab for treatment of inhalational anthrax was studied in a monkey model (Study 2) and a rabbit model (Studies 3 and 4) of inhalational anthrax disease. These 3 studies tested raxibacumab efficacy compared with placebo. Another study in a rabbit model (Study 1) evaluated the efficacy of raxibacumab in combination with an antibacterial drug relative to the antibacterial drug alone. Studies were randomized and blinded.
The animals were challenged with aerosolized B. anthracis spores (Ames strain) at 200 x LD50 to achieve 100% mortality if untreated. In rabbit Study 1, treatment was delayed until 84 hours after spore challenge. In monkey Study 2, study treatment commenced at the time of a positive serum electrochemiluminescence (ECL) assay for B. anthracis PA. The mean time between spore challenge and initiation of study treatment was 42 hours. In rabbit Studies 3 and 4, sustained elevation of body temperature above baseline for 2 hours or a positive result on serum ECL assay for PA served as the trigger for initiation of study treatment. The mean time between spore challenge and initiation of study treatment was 28 hours post-exposure. Efficacy in all therapeutic studies in animals was determined based on survival at the end of the study. Most study animals (88% to 100%) were bacteremic and had a positive ECL assay for PA prior to treatment in all 4 studies.
14.1 Treatment of Inhalational Anthrax in Combination with Antibacterial Drug
The efficacy of raxibacumab administered with levofloxacin as treatment of animals with systemic anthrax disease (84 hours after spore challenge) was evaluated in New Zealand White rabbits (Study 1). The dose of levofloxacin was chosen to yield a comparable exposure to that achieved by the recommended doses in humans. Levofloxacin and raxibacumab PK in this study were unaffected by product co-administration. Forty-two percent of challenged animals survived to treatment. Treatment with antibacterial drug plus raxibacumab resulted in 82% survival compared with 65% survival in rabbits treated with antibacterial drug alone, P = 0.0874 (Table 5).
Table 5. Survival Rates in NZW Rabbits in Combination Therapy Study, All-Treated Animals a Survival assessed 28 days after last dose of levofloxacin.
bP value based on a two-sided likelihood ratio chi-square test.
c 95% confidence interval based on normal approximation.Treatment Group
NZW Rabbits (35 days)a
Study 1Number (%) Survivors
P Valueb
95% CIc
Levofloxacin versus
Levofloxacin + Raxibacumab
Levofloxacin alone
24/37 (65%)
-
-
Levofloxacin + Raxibacumab 40 mg/kg intravenous single dose
32/39 (82%)
0.0874
(-2.4, 36.7)
14.2 Post-Exposure Prophylaxis/Early Treatment of Inhalational Anthrax
Monkey Study 2 and rabbit Studies 3 and 4 evaluated treatment with raxibacumab alone at an earlier time point after exposure than rabbit Study 1. Treatment with raxibacumab alone resulted in a statistically significant dose-dependent improvement in survival relative to placebo when administered at the time of initial manifestations of anthrax disease in the rabbit and monkey infection models (Table 6). Raxibacumab at 40 mg/kg intravenous single dose was superior to placebo in the rabbit and monkey studies in the all-treated and the bacteremic animal analysis populations. All surviving animals developed toxin-neutralizing antibodies.
Table 6. Survival Rates in Animals Treated with Raxibacumab, All-Treated Animals Cynomolgus Macaques
at 28 Daysa
Study 2NZW Rabbits
at 14 Daysb
Study 3NZW Rabbits
at 28 Daysa
Study 4a Survival measured at 28 days after spore challenge.
b Survival measured at 14 days after spore challenge.
cP value based on two-sided Fisher's exact test for comparisons between raxibacumab and placebo.
d 95% CIs are exact confidence intervals for the difference between raxibacumab and placebo.Treatment Group
Number (%) Survivors
P Valuec
95% CId
Number (%) Survivors
P Valuec
95% CId
Number (%) Survivors
P Valuec
95% CId
Placebo
0/12
0/17
0/24
20 mg/kg raxibacumab
7/14 (50%)
0.0064
(19.3, 73.7)
5/18 (28%)
0.0455
(6.6, 52.5)
-
-
-
40 mg/kg raxibacumab
9/14 (64%)
0.0007
(31.6, 84.7)
8/18 (44%)
0.0029
(21.3, 66.7)
11/24 (46%)
0.0002
(27.0, 66.1)
In other animal studies evaluating antibacterial drug alone and raxibacumab-antibacterial drug combination, the efficacy of an antibacterial drug alone (levofloxacin in rabbits and ciprofloxacin in monkeys) was very high (95% to 100%) when given at the initial manifestations of inhalational anthrax disease. The timing of treatment was similar to that reported for Studies 2, 3, and 4 above.
In another study, rabbits were exposed to 100 times LD50 B. anthracis spores and administered raxibacumab at a single dose of 40 mg/kg at the time of exposure, 12 hours, 24 hours, or 36 hours after exposure. Survival was 12/12 (100%) in animals treated at time of exposure or at 12 hours but decreased to 6/12 (50%) and 5/12 (42%) at 24 hours and 36 hours, respectively.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
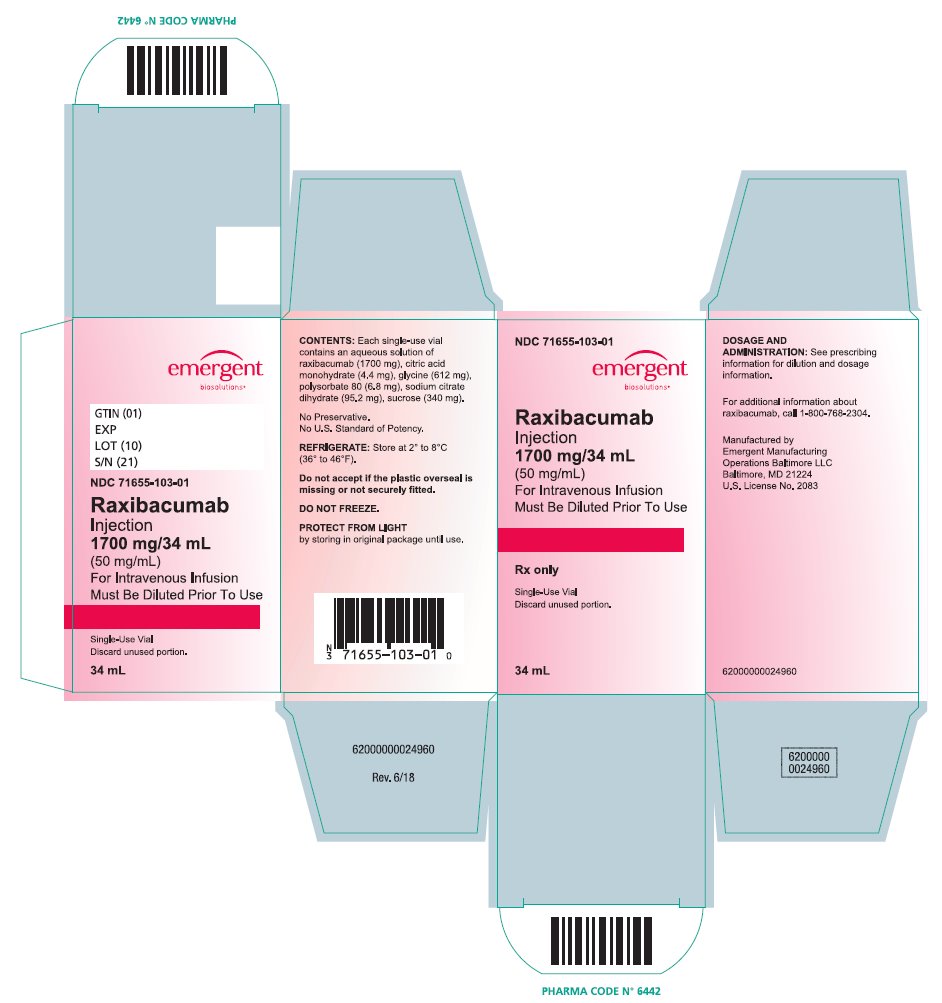
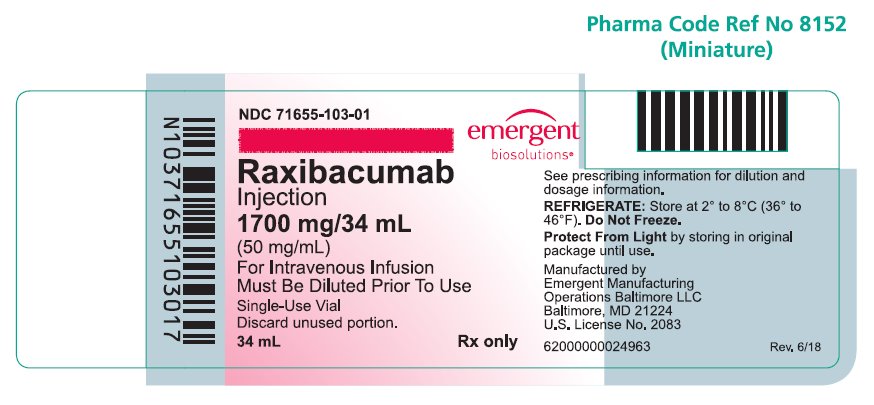
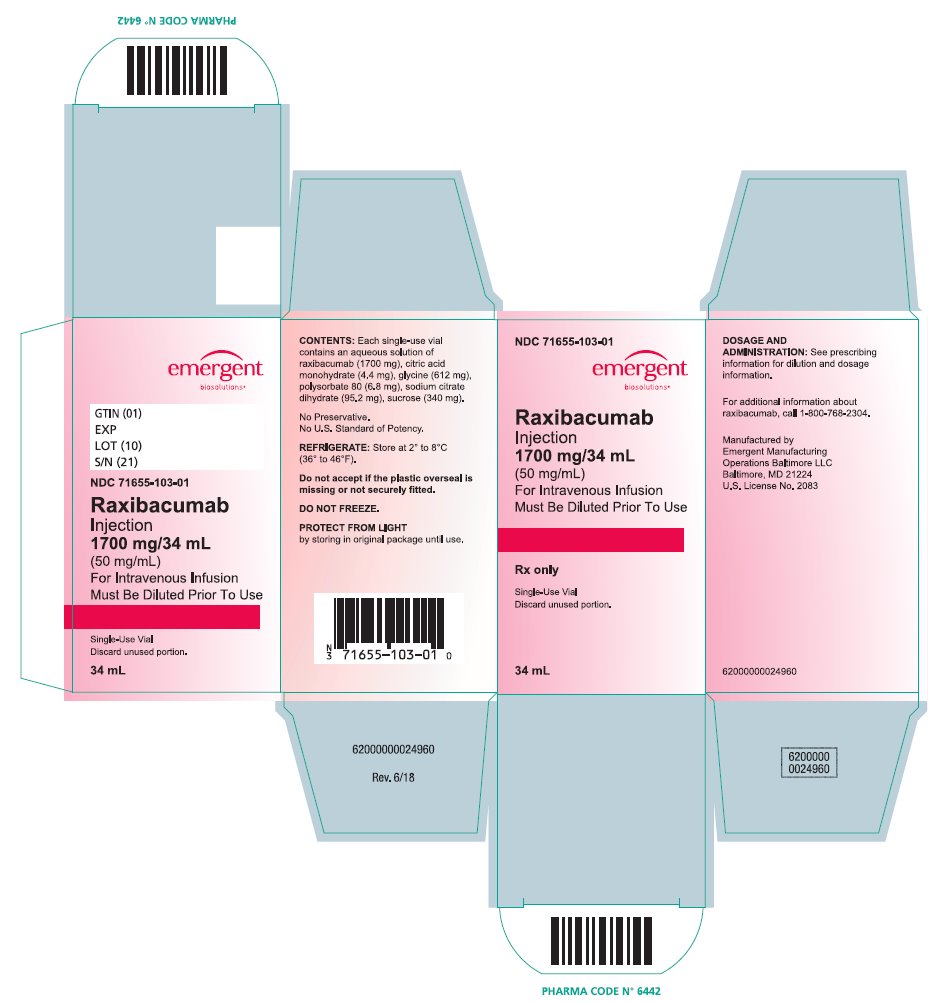
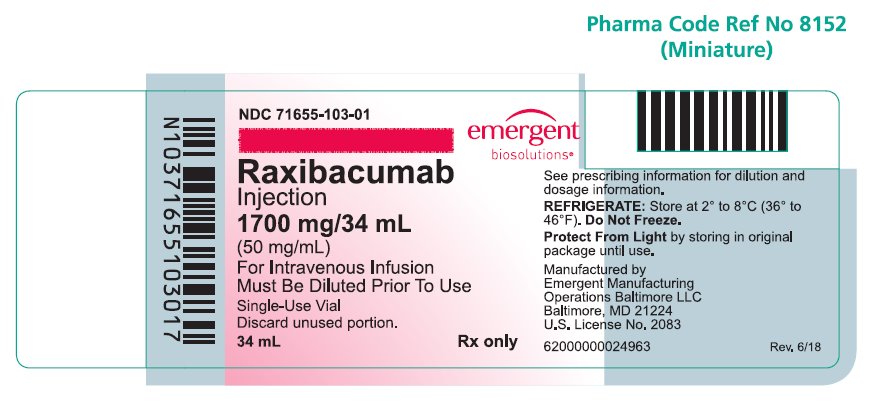
Raxibacumab is a sterile, preservative-free, clear to opalescent, colorless to pale yellow solution supplied in single-use vials containing 1,700 mg/34 mL (50 mg/mL) raxibacumab and is available in the following packaging configuration:
Single Unit Carton: Contains one single-use vial of raxibacumab 1,700 mg/34 mL (NDC 71655-103-01).
Raxibacumab must be refrigerated at 2°C to 8°C (36°F to 46°F). DO NOT FREEZE. Protect the vial from exposure to light, prior to use. Brief exposure to light, as with normal use, is acceptable. Store vial in original carton until time of use.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Efficacy Based on Animal Models
Inform patients that the efficacy of raxibacumab is based solely on efficacy studies demonstrating a survival benefit in animals and that the effectiveness of raxibacumab has not been tested in humans with anthrax. The safety of raxibacumab has been tested in healthy adults, but no safety data are available in children or pregnant women. Limited data are available in geriatric patients [see Use in Specific Populations (8.5)].
Hypersensitivity Reactions and Anaphylaxis
Inform patients that hypersensitivity reactions, including anaphylaxis, have occurred with the administration of raxibacumab. Inform patients of the signs and symptoms of hypersensitivity and anaphylaxis and instruct patients to seek immediate medical care if they experience such symptoms during or following administration of raxibacumab.
Prophylactic administration of diphenhydramine is recommended within 1 hour prior to administering raxibacumab to reduce the risk of occurrence and/or the severity of hypersensitivity reactions [see Warnings and Precautions (5.1)].
Manufactured by
Emergent Manufacturing Operations Baltimore LLC
Baltimore, MD 21224
U.S. License No. 2083Emergent BioSolutions Inc.
Any and all Emergent BioSolutions Inc. brand, product, service and feature names, logos and slogans are trademarks or registered trademarks of Emergent BioSolutions Inc. or its subsidiaries in the United States or other countries. All rights reserved.
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
RAXIBACUMAB (rack-see-BACK-u-mab)
Injection, for intravenous use
What is the most important information I should know about RAXIBACUMAB?
RAXIBACUMAB can cause serious side effects, including:- •
- Serious allergic reactions. Tell your healthcare provider right away if you have any of the following symptoms while receiving RAXIBACUMAB or after receiving RAXIBACUMAB:
- o
- rash
- o
- hives
- o
- itching
- o
- chills
- o
- chest tightness or shortness of breath
- o
- throat tightness or trouble breathing
- o
- swelling of the lips, throat, or tongue
- o
- feeling dizzy or lightheaded
What is RAXIBACUMAB?
- •
- RAXIBACUMAB is a prescription medicine used along with antibiotic medicines to treat people with inhalational anthrax. RAXIBACUMAB can also be used to prevent inhalational anthrax disease when there are no other treatment options.
- •
- The effectiveness of RAXIBACUMAB has been studied only in animals with inhalational anthrax. There have been no studies in people who have inhalational anthrax.
- •
- The safety of RAXIBACUMAB was studied in healthy adults. There have been no studies of RAXIBACUMAB in pediatric patients.
- •
- RAXIBACUMAB is not used for prevention or treatment of anthrax meningitis.
Before you receive RAXIBACUMAB, tell your healthcare provider about all of your medical conditions, including if you are:
- •
- allergic to any of the ingredients in RAXIBACUMAB. See the end of this leaflet for a list of the ingredients in RAXIBACUMAB.
- •
- allergic to diphenhydramine (BENADRYL).
- •
- pregnant or planning to become pregnant. It is not known if RAXIBACUMAB will harm your unborn baby.
- •
- breastfeeding or plan to breastfeed. It is not known if RAXIBACUMAB passes into your breast milk. You and your healthcare provider should decide if you will receive RAXIBACUMAB or breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I receive RAXIBACUMAB?
- •
- You will be given 1 dose of RAXIBACUMAB by a healthcare provider through a vein (intravenous infusion). It takes about 2 hours and 15 minutes to give you the full dose of medicine.
- •
- Your healthcare provider should give you a medicine called diphenhydramine (BENADRYL) before you receive RAXIBACUMAB to help reduce your chances of developing an allergic reaction from RAXIBACUMAB. BENADRYL may be given to you to take by mouth or through a vein.
- •
- BENADRYL may make you sleepy, and you should use caution if you will be driving or operating equipment.
What are the possible side effects of RAXIBACUMAB?
RAXIBACUMAB can cause serious side effects, including:
- •
- See “What is the most important information I should know about RAXIBACUMAB?”
The most common side effects of RAXIBACUMAB include:
- o
- rash
- o
- pain in your arms or legs
- o
- itchiness
- o
- sleepiness
- o
- injection site reactions, including redness and pain
- o
- headache
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of RAXIBACUMAB. For more information, ask your healthcare provider.
Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088. For more information go to dailymed.nlm.nih.gov.
General information about the safe and effective use of RAXIBACUMAB.
Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. This patient information leaflet summarizes the most important information about RAXIBACUMAB. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about RAXIBACUMAB that is written for health professionals.
What are the ingredients in RAXIBACUMAB?
Active ingredient: raxibacumab
Inactive ingredients: citric acid, glycine, polysorbate 80, sodium citrate, sucrose, Water for Injection.
Manufactured by Emergent Manufacturing Operations Baltimore LLC
Baltimore, MD 21224
U.S. License No. 2083
Emergent BioSolutions Inc.
For more information, go to www.emergentbiosolutions.com or call 1-800-768-2304.
BENADRYL is a trademark owned by or licensed to its owner and is not owned by or licensed to the Emergent group of companies. The maker of this brand is not affiliated with and does not endorse the Emergent group of companies or its products.
Any and all Emergent BioSolutions Inc. brand, product, service and feature names, logos and slogans are trademarks or registered trademarks of Emergent BioSolutions Inc. or its subsidiaries in the United States or other countries. All rights reserved.
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 6/2021
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 71655-103-01
Raxibacumab
Injection
1700 mg/34 mL
(50 mg/mL)
For Intravenous Infusion
Must be Diluted Prior To Use
Rx only
Single-Use Vial
Discard unused portion.
34 mL
See prescribing information for dilution and dosage information.
REFRIGERATE: Store at 2° to 8°C (36° to 46°F).
Do Not Freeze.
Protect From Light by storing in original package until use.
Manufactured by Emergent Manufacturing Operations Baltimore LLC, Baltimore, MD 21224
U.S. License No. 2083
Rev. 6/18
62000000024963
-
INGREDIENTS AND APPEARANCE
RAXIBACUMAB
raxibacumab injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:71655-103 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RAXIBACUMAB (UNII: 794PGL549S) (RAXIBACUMAB - UNII:794PGL549S) RAXIBACUMAB 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCINE (UNII: TE7660XO1C) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71655-103-01 1 in 1 CARTON 12/14/2012 1 34 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125349 12/14/2012 Labeler - Emergent Manufacturing Operations Baltimore LLC (968316831)