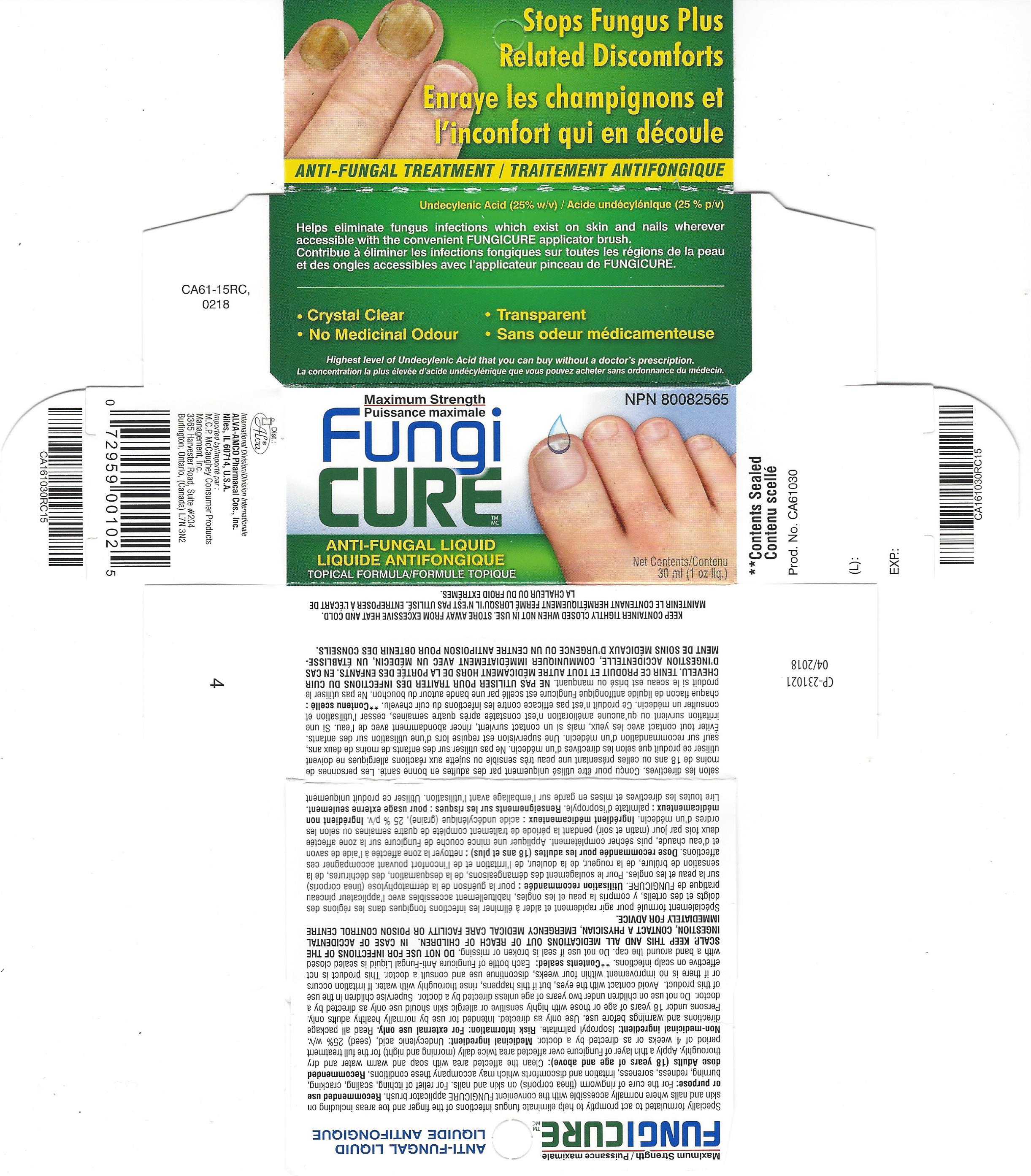
Label: FUNGICURE MAXIMUM STRENGTH- undecylenic acid liquid
- NDC Code(s): 52389-198-01, 52389-198-02, 52389-198-30, 52389-198-40
- Packager: Alva-Amco Pharmacal Companies, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated December 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FUNGICURE MAXIMUM STRENGTH
undecylenic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52389-198 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UNDECYLENIC ACID (UNII: K3D86KJ24N) (UNDECYLENIC ACID - UNII:K3D86KJ24N) UNDECYLENIC ACID 250 mg in 1 mL Inactive Ingredients Ingredient Name Strength ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52389-198-01 1 in 1 CARTON 07/20/2015 1 NDC:52389-198-30 30 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 2 NDC:52389-198-40 1 in 1 CARTON 06/06/2016 04/30/2022 2 NDC:52389-198-02 40 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/27/2005 Labeler - Alva-Amco Pharmacal Companies, Inc. (042074856)