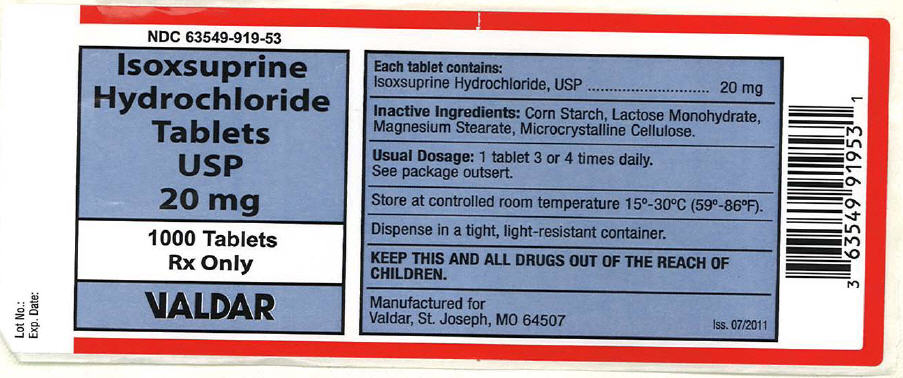
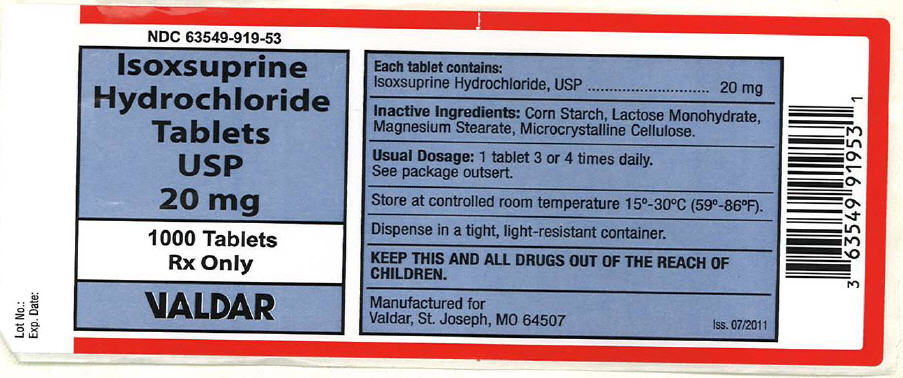
Label: ISOXSUPRINE HYDROCHLORIDE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 63549-919-53 - Packager: Vedco dba Valdar
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 2, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each tablet taken orally contains Isoxsuprine Hydrochloride, USP with the following chemical structure:
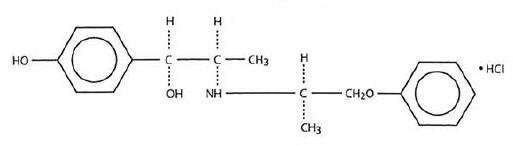
C18 H23 NO3 • HCl
p-Hydroxy-α[1-[(methyl-2-phenoxy-ethyl)amino]ethyl]benzyl alcohol hydrochloride.
-
INDICATIONS
Based on a review of this drug by the National Academy of Sciences-National Research and/or other information, the FDA has classified the indications as follows:
Possibly Effective
- For the relief of symptoms associated with cerebrovascular insufficiency.
- In peripheral vascular disease of arteriosclerosis obliterans, thromboangitis obliterans (Buerger's disease) and Raynaud's disease.
Final classification of the less-than-effective indications requires further investigation.
- CONTRAINDICATIONS
- PRECAUTIONS
-
ADVERSE REACTIONS
On rare occasion oral administration of the drug has been associated in time with the occurrence of hypotension, tachycardia, chest pain, nausea, vomiting, dizziness, abdominal distress, and severe rash. If rash appears, the drug should be discontinued.
Although available evidence suggests a temporal association of these reactions with Isoxsuprine Hydrochloride, a causal relationship can be neither confirmed nor refuted.
Beta Adrenergic receptor stimulants such as Isoxsuprine Hydrochloride have been used to inhibit pre-term labor.
Maternal and fetal tachycardia may occur under such use.
Hypocalcemia, hypoglycemia, hypotension and ileus have been reported to occur in infants whose mothers received Isoxsuprine Hydrochloride. Pulmonary edema has been reported in mothers treated with beta stimulants. Isoxsuprine Hydrochloride is neither approved nor recommended for use in the treatment of premature labor.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- COMPOSITION
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 20 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
ISOXSUPRINE HYDROCHLORIDE
isoxsuprine hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63549-919 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength isoxsuprine hydrochloride (UNII: V74TEQ36CO) (Isoxsuprine - UNII:R15UI3245N) isoxsuprine hydrochloride 20 mg Inactive Ingredients Ingredient Name Strength Lactose Monohydrate (UNII: EWQ57Q8I5X) Magnesium Stearate (UNII: 70097M6I30) Cellulose, Microcrystalline (UNII: OP1R32D61U) Starch, Corn (UNII: O8232NY3SJ) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code 20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63549-919-53 1000 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 08/23/2011 Labeler - Vedco dba Valdar (021634266)