Label: VIOKACE- pancrelipase tablet
- NDC Code(s): 73562-104-10, 73562-208-10
- Packager: Aimmune Therapeutics, Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VIOKACE safely and effectively. See full prescribing information for VIOKACE.
VIOKACE® (pancrelipase) tablets, for oral use
Initial U.S. Approval: 2012INDICATIONS AND USAGE
VIOKACE, in combination with a proton pump inhibitor, is indicated for the treatment of exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatectomy in adults. (1)
DOSAGE AND ADMINISTRATION
Important Dosing Information (2.1)
- •
- VIOKACE is a mixture of enzymes including lipases, proteases, and amylases and dosing is based on lipase units. Dosing scheme based on actual body weight or fat ingestion.
- •
- Individualize the dosage based on clinical symptoms, the degree of steatorrhea present, and the fat content of the diet.
- •
- Do not exceed 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/g fat ingested/day without further investigation. (5.1)
- •
- The total daily dosage should reflect approximately three meals plus two or three snacks per day. With each snack, administer approximately half the prescribed dose for a meal.
- •
- Do not substitute other pancreatic enzyme products for VIOKACE. When switching from another pancreatic enzyme product to VIOKACE, monitor patients for clinical symptoms of exocrine pancreatic insufficiency and titrate the dosage as needed.
Recommended Dosage
- •
- Adult Patients: The recommended initial starting dosage is 500 lipase units/kg/meal.
- •
- Titrate the dosage to 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/g fat ingested/day. Higher dosages may be administered if documented effective by fecal fat measures or improvement in malabsorption.
Preparation and Administration Instructions
DOSAGE FORMS AND STRENGTHS
Tablets (3):
- •
- 10,440 USP units of lipase; 39,150 USP units of protease; and 39,150 USP units of amylase
- •
- 20,880 USP units of lipase; 78,300 USP units of protease; and 78,300 USP units of amylase
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- •
- Fibrosing Colonopathy: Associated with high doses, usually over prolonged use and in pediatric patients with cystic fibrosis. Colonic stricture reported in pediatric patients less than 12 years of age with dosages exceeding 6,000 lipase units/kg/meal. Monitor during treatment for progression of preexisting disease. Do not exceed the recommended dosage, unless clinically indicated. (2.1, 5.1)
- •
- Irritation of the Oral Mucosa: May occur if tablets are crushed or chewed. (5.2)
- •
- Hyperuricemia: Reported with high dosages, consider monitoring blood uric acid levels in patients with gout, renal impairment, or hyperuricemia. (5.3)
- •
- Risk of Viral Transmission: The presence of porcine viruses that might infect humans cannot be definitely excluded. (5.4)
- •
- Hypersensitivity Reactions: Monitor patients with known reactions to proteins of porcine origin. If symptoms occur, initiate appropriate medical management; consider the risks and benefits of continued treatment. (5.5)
- •
- Potential for Exacerbation of Symptoms of Lactose Intolerance: tablets contain lactose monohydrate. Patients who have lactose intolerance may not be able to tolerate VIOKACE. (5.6)
ADVERSE REACTIONS
Most common adverse reactions (≥7%) are: anal pruritus and biliary tract stones. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Aimmune Therapeutics, Inc at 1-833-AIM2KNO (1-833-246-2566) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- •
- Pediatrics: VIOKACE use in pediatric patients may result in suboptimal weight gain, malnutrition and/or need for larger doses. The safety and effectiveness of VIOKACE in pediatric patients have not been established. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
2.2 Recommended Dosage
2.3 Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Fibrosing Colonopathy
5.2 Irritation of the Oral Mucosa
5.3 Hyperuricemia
5.4 Risk of Viral Transmission
5.5 Hypersensitivity Reactions
5.6 Potential for Exacerbation of Symptoms of Lactose Intolerance
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
VIOKACE is a mixture of enzymes including lipases, proteases, and amylases. VIOKACE dosing is based on lipase units.
- •
- Administer VIOKACE with a proton pump inhibitor.
- •
- Use either an actual body weight or fat ingestion‑based dosing scheme.
- •
- Start at the lowest recommended dosage and individualize the dosage based on clinical symptoms, the degree of steatorrhea present, and the fat content of the diet. Changes in dosage may require an adjustment period of several days.
- •
- Do not exceed 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/g fat ingested/day without further investigation [see Warnings and Precautions (5.1)]. Higher dosages may be administered if they are documented to be effective by fecal fat measures or an improvement in signs or symptoms of malabsorption including measures of nutritional status.
- •
- The total daily dosage should reflect approximately three meals plus two or three snacks per day. With each snack, administer approximately half the prescribed VIOKACE dose for a meal.
- •
- Do not substitute other pancreatic enzyme products for VIOKACE. When switching from another pancreatic enzyme product to VIOKACE, monitor patients for clinical symptoms of exocrine pancreatic insufficiency and titrate the dosage as needed.
2.2 Recommended Dosage
Adult Patients:
The recommended oral initial starting dosage of VIOKACE is 500 lipase units/kg/meal.
If signs and symptoms of malabsorption persist, increase the dosage. Titrate to a maximum of either 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/grams of fat ingested/day.
2.3 Preparation and Administration Instructions
Adult Patients:
- •
- Take VIOKACE with meals or snacks. If a dose is missed, take the next dose with the next meal or snack.
- •
- Swallow tablets whole. Do not crush or chew VIOKACE tablets.
- •
- Consume sufficient liquids (water) to ensure complete swallowing of VIOKACE tablets [see Warnings and Precautions (5.2)].
-
3 DOSAGE FORMS AND STRENGTHS
Tablets are available in the following strengths:
- •
- 10,440 USP units of lipase; 39,150 USP units of protease; and 39,150 USP units of amylase as a tan, round, biconvex tablet with VIO9111 engraved on one side and 9111 on the other side.
- •
- 20,880 USP units of lipase; 78,300 USP units of protease; and 78,300 USP units of amylase as a tan, oval, biconvex tablet with V16 engraved on one side and 9116 on the other side.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Fibrosing Colonopathy
Fibrosing colonopathy has been reported following treatment with pancreatic enzyme products. Fibrosing colonopathy is a rare, serious adverse reaction initially described in association with use of high-dose pancreatic enzyme products, usually over a prolonged period of time and most commonly reported in pediatric patients with cystic fibrosis. Pancreatic enzyme products exceeding 6,000 lipase units/kg/meal have been associated with colonic stricture, a complication of fibrosing colonopathy, in pediatric patients less than 12 years of age. The underlying mechanism of fibrosing colonopathy remains unknown.
If there is a history of fibrosing colonopathy, monitor patients during treatment with VIOKACE because some patients may be at risk of progressing to colonic stricture formation. It is uncertain whether regression of fibrosing colonopathy occurs. Do not exceed the recommended dosage of either 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or 4,000 lipase units/g fat ingested/day without further investigation. Higher dosages may be administered if they are documented to be effective by fecal fat measures or an improvement in signs or symptoms of malabsorption including measures of nutritional status. Patients receiving dosages higher than 6,000 lipase units/kg/meal should frequently monitored for symptoms of fibrosing colonopathy and the dosage decreased or titrated downward to a lower range if clinically appropriate [see Dosage and Administration (2.1)].
5.2 Irritation of the Oral Mucosa
Crushing or chewing VIOKACE tablets can result in irritation of the oral mucosa, and/or loss of enzyme activity. Instruct the patient to swallow tablets whole. Do not crush or chew VIOKACE tablets. Consume sufficient liquids immediately following administration of VIOKACE to ensure complete swallowing [see Dosage and Administration (2.3)].
5.3 Hyperuricemia
Pancreatic enzyme products contain purines that may increase blood uric acid levels. High dosages have been associated with hyperuricosuria and hyperuricemia [see Overdosage (10)].
Consider monitoring blood uric acid levels in patients with gout, renal impairment, or hyperuricemia during treatment with VIOKACE.
5.4 Risk of Viral Transmission
VIOKACE is sourced from pancreatic tissue from pigs used for food consumption. Although the risk that VIOKACE will transmit an infectious agent to humans has been reduced by testing for certain viruses during manufacturing and by inactivating certain viruses during manufacturing, there is a theoretical risk for transmission of viral disease, including diseases caused by novel or unidentified viruses. Thus, the presence of porcine viruses that might infect humans cannot be definitely excluded. However, no cases of transmission of an infectious illness associated with the use of porcine pancreatic extracts have been reported.
5.5 Hypersensitivity Reactions
Severe hypersensitivity reactions including anaphylaxis, asthma, hives, and pruritus have been reported with pancreatic enzyme products [see Adverse Reactions (6.2)]. If symptoms occur, initiate appropriate medical management.
Monitor patients with a known hypersensitivity reaction to proteins of porcine origin for hypersensitivity reactions during treatment with VIOKACE. The risks and benefits of continued VIOKACE treatment in patients with severe hypersensitivity reactions should be taken into consideration with the overall clinical needs of the patient.
5.6 Potential for Exacerbation of Symptoms of Lactose Intolerance
VIOKACE tablets contain lactose monohydrate [see Description (11)]. Patients who have lactose intolerance may not be able to tolerate VIOKACE.
-
6 ADVERSE REACTIONS
The following serious or otherwise important adverse reactions are described elsewhere in the labeling:
- •
- Fibrosing Colonopathy [see Warnings and Precautions (5.1)]
- •
- Irritation of the Oral Mucosa [see Warnings and Precautions (5.2)]
- •
- Hyperuricemia [see Warnings and Precautions (5.3)]
- •
- Risk of Viral Transmission [see Warnings and Precautions (5.4)]
- •
- Hypersensitivity Reactions [see Warnings and Precautions (5.5)]
- •
- Potential for Exacerbation of Symptoms of Lactose Intolerance [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to VIOKACE in 30 adult patients with exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatectomy in a single, multicenter, randomized, parallel, placebo-controlled, double-blind study [see Clinical Studies (14)].
Adverse reactions that were reported in at least 2 VIOKACE-treated patients (greater than or equal to 7%) are shown in Table 1. There were no adverse reactions reported in two or more patients in the placebo group (N=20).
Table 1: Adverse Reactions* in a Clinical Trial of Adult Patients with Exocrine Pancreatic Insufficiency due to Chronic Pancreatitis or Pancreatectomy - *
- Reported in at least 2 VIOKACE-treated patients (greater than or equal to 7%) and at a higher rate than placebo-treated patients.
Adverse Reaction
VIOKACE N = 30 (%)
Anal pruritus
2 (7%)
Biliary tract stones
2 (7%)
The following adverse reactions were reported in one VIOKACE-treated patient each: anemia, abdominal pain, ascites, flatulence, headache, hydocholecystis, peripheral edema, rash, renal cyst, and viral infection.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of VIOKACE or other pancreatic enzyme products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Eye Disorders
- •
- blurred vision
Gastrointestinal Disorders
- •
- fibrosing colonopathy and distal intestinal obstruction syndrome
- •
- abdominal pain, diarrhea, flatulence, constipation, and nausea
Immune System Disorders
- •
- anaphylaxis, asthma, hives and pruritis
Investigations
- •
- asymptomatic elevations of liver enzymes
Musculoskeletal System
- •
- myalgia, muscle spasm
Skin and Subcutaneous Tissue Disorders
- •
- urticaria and rash
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Published data from case reports with pancrelipase use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. Pancrelipase is minimally absorbed systematically; therefore, maternal use is not expected to result in fetal exposure to the drug. Animal reproduction studies have not been conducted with pancrelipase.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of pancrelipase in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. Pancrelipase is minimally absorbed systemically following oral administration, therefore maternal use is not expected to result in clinically relevant exposure of breastfed infants to the drug. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VIOKACE and any potential adverse effects on the breastfed child from VIOKACE or from the underlying maternal conditions.
8.4 Pediatric Use
The safety and effectiveness of VIOKACE in pediatric patients have not been established.
Use of VIOKACE in pediatric patients may increase the risk of inadequate treatment of pancreatic insufficiency and result in suboptimal weight gain, malnutrition and/or need for larger doses of pancreatic enzyme replacement due to tablet degradation in the gastric environment of the stomach.
High dosages of pancreatic enzyme products have been associated with fibrosing colonopathy and colonic strictures in pediatric patients less than 12 years of age [see Warnings and Precautions (5.1)].
8.5 Geriatric Use
Clinical studies of VIOKACE did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between patients aged 65 years and over and younger adult patients.
-
10 OVERDOSAGE
Chronic high dosages of pancreatic enzyme products have been associated with fibrosing colonopathy and colonic strictures [see Warnings and Precautions (5.1)]. High dosages of pancreatic enzyme products have been associated with hyperuricosuria and hyperuricemia [see Warnings and Precautions (5.3)].
-
11 DESCRIPTION
Pancrelipase is a pancreatic enzyme product consisting of a mixture of enzymes including lipases, proteases, and amylases, and is an extract derived from porcine pancreatic glands.
VIOKACE (pancrelipase) tablets are for oral administration and available as follows:
- •
- 10,440 USP units of lipase; 39,150 USP units of protease; and 39,150 USP units of amylase as a tan, round biconvex tablet and have VIO9111 engraved on one side and 9111 on the other side.
- •
- 20,880 USP units of lipase; 78,300 USP units of protease; and 78,300 USP units of amylase as a tan, oval, biconvex tablet with V16 engraved on one side and 9116 on the other side.
Inactive ingredients in VIOKACE include colloidal silicon dioxide, crosscarmellose sodium, lactose monohydrate, microcrystalline cellulose, stearic acid and talc.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pancreatic enzyme products contain a mixture of lipases, proteases, and amylases that catalyze the hydrolysis of fats to monoglycerides, glycerol and free fatty acids, proteins into peptides and amino acids, and starches into dextrins and short chain sugars such as maltose and maltriose in the duodenum and proximal small intestine, thereby acting like digestive enzymes physiologically secreted by the pancreas.
12.2 Pharmacodynamics
For patients consuming a high fat diet in the clinical trial, the coefficient of fat absorption (CFA) was higher in patients who received VIOKACE compared to the placebo treatment group, indicating improved fat absorption [see Clinical Studies (14)].
12.3 Pharmacokinetics
Following oral administration, the lipases, proteases, and amylases released from VIOKACE are not absorbed from the gastrointestinal tract in appreciable amounts.
Drug Interactions
The lipases, proteases, and amylases of VIOKACE are not substrates of CYP enzymes or transporters. CYP enzymes or transporters mediated drug interactions are not expected.
-
14 CLINICAL STUDIES
A randomized, double-blind, placebo-controlled, parallel group study was conducted in 50 adult patients, aged 24 to 70 years, with exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatectomy. Eighteen patients had a history of pancreatectomy (11 were treated with VIOKACE). All patients were maintained on a controlled high fat diet of 100 grams of fat per day. After a washout period (6 to 7 days), patients were randomized to a fixed dose of VIOKACE or placebo, in combination with a proton pump inhibitor: 125,280 lipase units per meal (3 meals) and 41,760 lipase units per snack (2 snacks) (6 tablets per meal and 2 tablets per snacks) ). Forty-nine patients completed the double-blind treatment period (6 to 7 days); 29 patients received VIOKACE, and 20 patients received placebo. Duration of exposure to VIOKACE ranged from 6 to 7 days. The majority of the subjects were White (96%) and male (82%).
Coefficient of Fat Absorption Endpoint and Results
The coefficient of fat absorption (CFA) was determined by a 72-hour stool collection during both the washout period and end of double-blind period when both fat excretion and fat ingestion were measured. The mean change in CFA at the end of the double-blind treatment period in the VIOKACE and placebo groups is shown in Table 2.
Table 2: Change in Coefficient of Fat Absorption in Adults with Exocrine Pancreatic Insufficiency Due to Chronic Pancreatitis and Pancreatectomy - Intent to Treat Population - *
- p<0.0001
VIOKACE
n = 30Placebo
n = 20CFA [%]
Washout Period (Mean, SD)
48 (24)
57 (22)
End of Double-Blind Period (Mean, SD)
86 (9)
58 (24)
Change in CFA** [%]
Treatment Difference (95% CI)
28 (18,37)
Subgroup analyses of the CFA results showed that mean change in CFA with VIOKACE treatment (from the washout period to the end of the double-blind period) was greater in patients with lower wash-out period CFA values than in patients with higher wash-out period CFA values.
Only 2 of the patients with a history of total pancreatectomy were treated with VIOKACE. One of these patients had a CFA of 12% during the wash-out period and a CFA of 90% at the end of the double-blind period; the other patient had a CFA of 38% during the wash-out period and a CFA of 77% at the end of the double-blind period. The remaining 9 patients with a history of partial pancreatectomy treated with VIOKACE had a mean CFA of 56% during the wash-out period and a mean CFA of 86% at the end of the double-blind period.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
VIOKACE (pancrelipase) tablets are supplied as follows:
Strength
Description
Supplied As
NDC Number
10,440 USP units of lipase; 39,150 USP units of protease; 39,150 USP units of amylase
tan, round, biconvex tablet with VIO9111 engraved on one side and 9111 on the other side
bottles of 100
73562-104-10
20,880 USP units of lipase; 78,300 USP units of protease; 78,300 USP units of amylase
tan, oval, biconvex tablet with V16 engraved on one side and 9116 on the other side
bottles of 100
73562-208-10
Storage and Handling
- •
- Store VIOKACE at room temperature 20°C to 25°C (68°F to 77°F), and protect from moisture. Brief excursions permitted up to 40°C (104°F) for 24 hours. After opening, keep the container tightly closed between uses to protect from moisture.
- •
- Store and dispense VIOKACE in the original container.
- •
- VIOKACE is dispensed in bottles containing a desiccant.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide).
Fibrosing Colonopathy
Advise patients that if signs and symptoms of colon stricture formation occur (e.g., stomach area (abdominal) pain, bloating, trouble passing stool (constipation), nausea, vomiting, diarrhea) to immediately contact their healthcare provider [see Warnings and Precautions (5.1)].
Hyperuricemia
Advise the patient that hyperuricemia may occur in patients with gout or renal impairment and to contact the healthcare provider if they experience pain, stiffness, redness or swelling of their joints [see Warnings and Precautions (5.3)].
Hypersensitivity Reactions
Inform the patient that severe hypersensitivity reactions, including anaphylaxis asthma, hives, and pruritus, have been reported with use of pancreatic enzyme products. Seek medical attention if signs or symptoms of a hypersensitivity reaction develop [see Warnings and Precautions (5.5)].
Dosage
Advise the patient to increase take VIOKACE as prescribed, and to contact the healthcare provider if signs and symptoms of malabsorption persist [see Dosage and Administration (2.1)].
Administration
Instruct the patient to:
- •
- Take VIOKACE with meals or snacks.
- •
- Swallow tablets whole. Do not crush or chew VIOKACE tablets.
- •
- Consume sufficient liquids (water) to ensure complete swallowing of VIOKACE tablets.
Storage
Instruct the patient or caregiver as follows:
- •
- Keep VIOKACE in a dry place and protect from moisture and heat.
- •
- After opening, keep the container tightly closed between uses to protect from moisture.
- •
- Keep VIOKACE in the original bottle.
- •
- The desiccant packet should not be eaten or thrown away.
-
Medication Guide
MEDICATION GUIDE
VIOKACE ® (vye-oh-kase)
(pancrelipase)Tablets, for oral use
What is the most important information I should know about VIOKACE?
VIOKACE may increase your chance of having a rare bowel disorder called fibrosing colonopathy especially if taken at a high dose for a long time in children with cystic fibrosis. This condition is serious and may require surgery. The risk of having this condition may be reduced by following the dosing instructions that your doctor gave you.
Call your doctor right away if you have any unusual or severe:
- •
- stomach area (abdominal) pain
- •
- bloating
- •
- trouble passing stool (having bowel movements)
- •
- nausea, vomiting, or diarrhea
Take VIOKACE exactly as prescribed by your doctor. Do not take more VIOKACE than directed by your doctor.
What is VIOKACE?
- •
- VIOKACE is a prescription medicine used with a proton pump inhibitor medicine (PPI) to treat adults who cannot digest food normally. Adults with swelling of the pancreas that lasts a long time (chronic pancreatitis), or who have had some or all of their pancreas removed (pancreatectomy) may not digest food normally because their pancreas does not make enough enzymes.
- •
- VIOKACE contains a mixture of digestive enzymes (including lipases, proteases, and amylases) from pig pancreas.
- •
- It is not known if VIOKACE is safe and effective in children. Use of VIOKACE in children may result in poor nutrition and slowing of growth.
Before taking VIOKACE, tell your doctor about all your medical conditions, including if you:
- •
- are allergic to pork (pig) products.
- •
- have a history of blockage of your intestines, or scarring or thickening of your bowel wall (fibrosing colonopathy).
- •
- have gout, kidney disease, or a condition called high blood uric acid (hyperuricemia).
- •
- have trouble swallowing tablets.
- •
- are lactose intolerant.
- •
- have any other medical condition.
- •
- are pregnant or plan to become pregnant.
- •
- are breastfeeding or plan to breastfeed. It is not known if VIOKACE passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take VIOKACE.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
How should I take VIOKACE?
- •
- Take VIOKACE tablets exactly as your doctor tells you. Contact your doctor if you continue to have signs and symptoms of malabsorption (not absorbing nutrients from food) such as abdominal pain, abdominal distention, bloating, fatty stools, or weight loss. Your dose may need to be changed.
- •
- You should not switch VIOKACE with any other pancreatic enzyme product without first talking to your doctor.
- •
- Do not take more tablets in a day than the number your doctor tells you to take (total daily dose).
- •
- Always take VIOKACE with a meal or a snack and enough liquid (water) to swallow VIOKACE completely. If you eat a lot of meals or snacks in a day, be careful not to go over your total daily dose.
- •
- Your doctor may change your dose based on the amount of fatty foods you eat or based on your weight.
- •
- Your doctor should also prescribe a medicine for you called a proton pump inhibitor (PPI) to decrease stomach acid. VIOKACE should be taken with a PPI to help prevent VIOKACE from breaking down in your stomach.
- •
- Swallow VIOKACE tablets whole. Do not crush or chew the tablets. Be careful to make sure that no VIOKACE is left in your mouth. Crushing or chewing the VIOKACE tablets may cause irritation in your mouth, or change the way VIOKACE works in your body.
- •
- If you forget to take VIOKACE, wait until your next meal and take your usual number of tablets. Take your next dose at your usual time. Do not take two doses at one time.
What are the possible side effects of VIOKACE?
VIOKACE may cause serious side effects, including:
- •
- See “What is the most important information I should know about VIOKACE?”
- •
- Irritation of the inside of your mouth. This can happen if VIOKACE is not swallowed completely.
- •
- Increase in blood uric acid levels (hyperuricemia). This may happen in people with gout, kidney problems, or those who take high doses of pancrelipase, the active ingredient in VIOKACE. Call your doctor if you have pain, stiffness, redness or swelling of your joints.
- •
- Severe allergic reactions. Severe allergic reactions have happened in people taking pancreatic enzyme products like VIOKACE. Stop taking VIOKACE and get emergency treatment right away if you have any of these symptoms: trouble with breathing, skin rashes, swollen lips or itching.
The most common side effects of VIOKACE include:
- •
- you can develop stones that form in your gallbladder and the tubes that carry bile to your small intestines.
- •
- anal itching
Other Possible Side Effects:
VIOKACE and other pancreatic enzyme products are made from the pancreas of pigs, the same pigs people eat as pork. These pigs may carry viruses. Although it has never been reported, it may be possible for a person to get a viral infection from taking pancreatic enzyme products that come from pigs.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possibleside effects of VIOKACE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Aimmune Therapeutics, Inc at 1-833-AIM2KNO (1-833-246-2566)
How should I store VIOKACE?
- •
- Store VIOKACE at room temperature between 68°F to 77°F (20°C to 25°C). Avoid heat.
- •
- Keep VIOKACE in a dry place and in its original container.
- •
- After opening the bottle, keep it closed tightly between uses to protect from moisture.
- •
- The VIOKACE bottle contains a desiccant packet to help keep your medicine dry (protect it from moisture). Do not eat or throw away the desiccant packet.
Keep VIOKACE and all medicines out of the reach of children.
General information about the safe and effective use of VIOKACE.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use VIOKACE for a condition for which it was not prescribed. Do not give VIOKACE to other people to take, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or doctor for information about VIOKACE that is written for health professionals.
What are the ingredients in VIOKACE?
Active ingredients: lipase, protease and amylase
Inactive ingredients: colloidal silicon dioxide, crosscarmellose sodium, lactose monohydrate, microcrystalline cellulose, stearic acid and talc.
Manufactured by:
Viokace LLC
1007 US Highway 202/206,
Bridgewater, NJ 08807, USA
US License No. 2196
Manufactured for:
Aimmune Therapeutics, Inc.
Bridgewater, NJ 08807, USA
For more information, please call Aimmune Therapeutics, Inc at 1-833-AIM2KNO (1-833-246-2566).
All trademarks are owned by Société des Produits Nestlé S.A., Vevey, Switzerland or used with permission.
©2024 Nestlé.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised: 02/2024 -
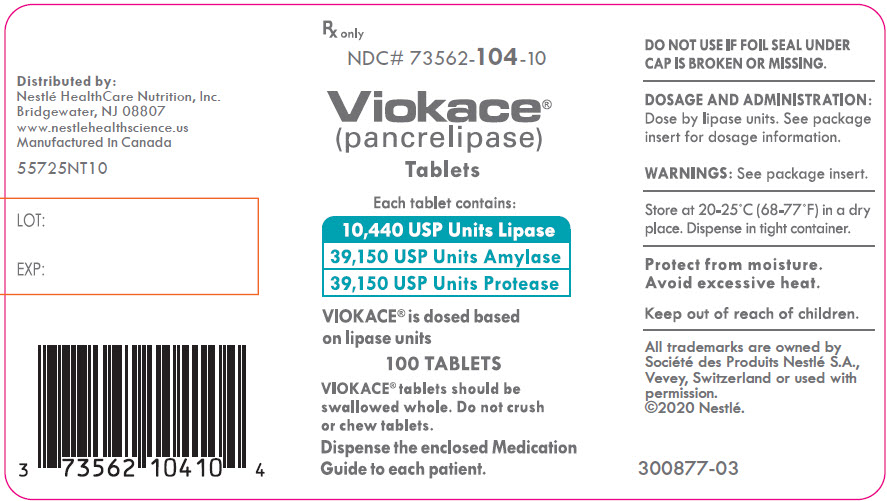
PRINCIPAL DISPLAY PANEL - Lipase 10,440 USP Units
Rx only
NDC# 73562-104-10
Viokace®
(pancrelipase)
TabletsEach tablet contains:
10,440 USP Units Lipase
39,150 USP Units Amylase
39,150 USP Units ProteaseVIOKACE® is dosed based
on lipase units100 TABLETS
VIOKACE® tablets should be
swallowed whole. Do not crush
or chew tablets.Dispense the enclosed Medication
Guide to each patient. -
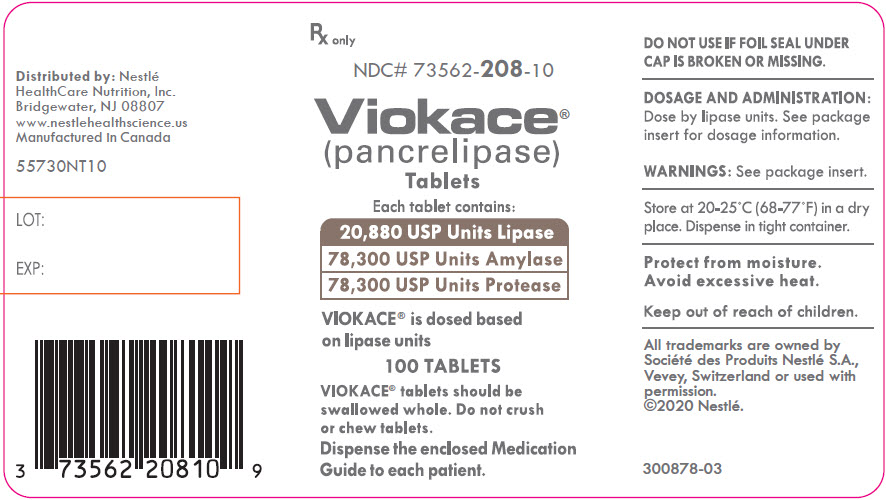
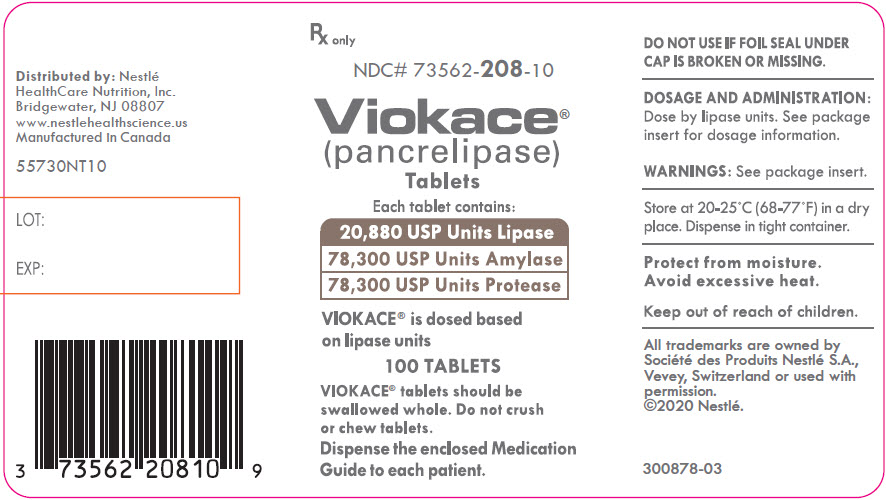
PRINCIPAL DISPLAY PANEL - Lipase 20,880 USP Units
Rx only
NDC# 73562-208-10
Viokace®
(pancrelipase)
TabletsEach tablet contains:
20,880 USP Units Lipase
78,300 USP Units Amylase
78,330 USP Units ProteaseVIOKACE® is dosed based
on lipase units100 TABLETS
VIOKACE® tablets should be
swallowed whole. Do not crush
or chew tablets.Dispense the enclosed Medication
Guide to each patient. -
INGREDIENTS AND APPEARANCE
VIOKACE
pancrelipase tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73562-104 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANCRELIPASE LIPASE (UNII: 8MYC33932O) (PANCRELIPASE LIPASE - UNII:8MYC33932O) PANCRELIPASE LIPASE 10440 [USP'U] PANCRELIPASE PROTEASE (UNII: 3560D81V50) (PANCRELIPASE PROTEASE - UNII:3560D81V50) PANCRELIPASE PROTEASE 39150 [USP'U] PANCRELIPASE AMYLASE (UNII: YOJ58O116E) (PANCRELIPASE AMYLASE - UNII:YOJ58O116E) PANCRELIPASE AMYLASE 39150 [USP'U] Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) STEARIC ACID (UNII: 4ELV7Z65AP) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color BROWN (Tan) Score no score Shape ROUND (Biconvex) Size 13mm Flavor Imprint Code VIO9111;9111 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73562-104-10 1 in 1 CARTON 03/01/2021 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022542 03/01/2021 VIOKACE
pancrelipase tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73562-208 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANCRELIPASE LIPASE (UNII: 8MYC33932O) (PANCRELIPASE LIPASE - UNII:8MYC33932O) PANCRELIPASE LIPASE 20880 [USP'U] PANCRELIPASE PROTEASE (UNII: 3560D81V50) (PANCRELIPASE PROTEASE - UNII:3560D81V50) PANCRELIPASE PROTEASE 78300 [USP'U] PANCRELIPASE AMYLASE (UNII: YOJ58O116E) (PANCRELIPASE AMYLASE - UNII:YOJ58O116E) PANCRELIPASE AMYLASE 78300 [USP'U] Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) STEARIC ACID (UNII: 4ELV7Z65AP) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color BROWN (Tan) Score no score Shape OVAL (Biconvex) Size 19mm Flavor Imprint Code V16;9116 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73562-208-10 1 in 1 CARTON 03/01/2021 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA022542 03/01/2021 Labeler - Aimmune Therapeutics, Inc (057562771)