Label: MOTOFEN- difenoxin and atropine sulfate tablet
- NDC Code(s): 54766-200-04, 54766-200-10
- Packager: Sebela Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: New Drug Application
Drug Label Information
Updated December 14, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Each five-sided dye free MOTOFEN® tablet contains: 1 mg of difenoxin (equivalent to 1.09 mg of difenoxin hydrochloride) and 0.025 mg of atropine sulfate (equivalent to 0.01 mg of atropine).
Difenoxin hydrochloride, 1-(3-cyano-3,3-diphenylpropyl)-4-phenyl-4-piperidinecarboxylic acid monohydrochloride, is an orally administered antidiarrheal agent which is chemically related to the narcotic meperidine.The structural formula is:
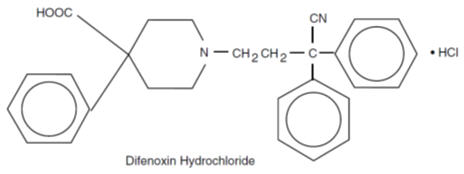
Atropine sulfate is present to discourage deliberate overdose.
Atropine sulfate, an anticholinergic, is benzeneacetic acid, α-(hydroxymethyl)-, 8-methyl-8-azabicyclo[3.2.1]oct-3-yl ester, endo-(±)-, (2:1) (salt), monohydrate and has the following structural formula:
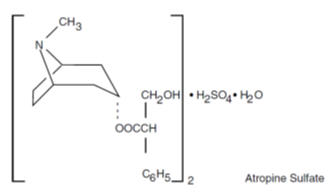
Inactive Ingredients: calcium stearate, cellulose, lactose, corn starch.
-
CLINICAL PHARMACOLOGY
Animal studies have shown that difenoxin hydrochloride manifests its antidiarrheal effect by slowing intestinal motility. The mechanism of action is by a local effect on the gastrointestinal wall.
Difenoxin is the principal active metabolite of diphenoxylate.
Following oral administration of MOTOFEN®, difenoxin is rapidly and extensively absorbed. Mean peak plasma levels of approximately 160 ng/mL occurred within 40 to 60 minutes in most patients following an oral dose of 2 mg. Plasma levels decline to less than 10% of their peak values within 24 hours and to less than 1% of their peak values within 72 hours. This decline parallels the appearance of difenoxin and its metabolites in the urine. Difenoxin is metabolized to an inactive hydroxylated metabolite. Both the drug and its metabolites are excreted, mainly as conjugates, in urine and feces.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
MOTOFEN® is contraindicated in patients with diarrhea associated with organisms that penetrate the intestinal mucosa (toxigenic E. coli, Salmonella species, Shigella) and pseudomembranous colitis associated with broad spectrum antibiotics. Antiperistaltic agents should not be used in the conditions because they may prolong and/or worsen diarrhea.
MOTOFEN® is contraindicated in children under 2 years of age because of the decreased margin of safety of drugs in this class in younger age groups.
MOTOFEN® is contraindicated in patients with a known hypersensitivity to difenoxin, atropine, or any of the inactive ingredients, and in patients who are jaundiced.
-
WARNINGS
MOTOFEN® IS NOT AN INNOCUOUS DRUG AND DOSAGE RECOMMENDATIONS SHOULD BE STRICTLY ADHERED TO. MOTOFEN® IS NOT RECOMMENDED FOR CHILDREN UNDER 2 YEARS OF AGE. OVERDOSAGE MAY RESULT IN SEVERE RESPIRATORY DEPRESSION AND COMA, POSSIBLY LEADING TO PERMANENT BRAIN DAMAGE OR DEATH (SEE OVERDOSAGE). THEREFORE, KEEP THIS MEDICATION OUT OF THE REACH OF CHILDREN.
FLUID AND ELECTROLYTE BALANCE – THE USE OF MOTOFEN® DOES NOT PRECLUDE THE ADMINISTRATION OF APPROPRIATE FLUID AND ELECTROLYTE THERAPY. DEHYDRATION, PARTICULARLY IN CHILDREN, MAY FURTHER INFLUENCE THE VARIABILITY OF RESPONSE TO MOTOFEN® AND MAY PREDISPOSE TO DELAYED DIFENOXIN INTOXICATION. DRUG-INDUCED INHIBITION OF PERISTALSIS MAY RESULT IN FLUID RETENTION IN THE COLON, AND THIS MAY FURTHER AGGRAVATE DEHYDRATION AND ELECTROLYTE IMBALANCE.
IF SEVERE DEHYDRATION OR ELECTROLYTE IMBALANCE IS MANIFESTED, MOTOFEN® SHOULD BE WITHHELD UNTIL APPROPRIATE CORRECTIVE THERAPY HAS BEEN INITIATED.
Ulcerative Colitis – In some patients with acute ulcerative colitis, agents which inhibit intestinal motility or delay intestinal transit time have been reported to induce toxic megacolon. Consequently, patients with acute ulcerative colitis should be carefully observed and MOTOFEN® therapy should be discontinued promptly if abdominal distention occurs or if other untoward symptoms develop.
Liver and Kidney Disease – MOTOFEN® should be used with extreme caution in patients with advanced hepatorenal disease and in all patients with abnormal liver function tests since hepatic coma may be precipitated.
Atropine – A subtherapeutic dose of atropine has been added to difenoxin hydrochloride to discourage deliberate overdosage. Usage of MOTOFEN® in recommended doses is not likely to cause prominent anticholinergic side effects, but MOTOFEN® should be avoided in patients in whom anticholinergic drugs are contraindicated. The warnings and precautions for use of anticholinergic agents should be observed. In children, signs of atropinism may occur even with recommended doses of MOTOFEN®, particularly in patients with Down’s Syndrome. -
PRECAUTIONS
CAUTION PATIENTS TO ADHERE STRICTLY TO RECOMMENDED DOSAGE SCHEDULES. THE MEDICATION SHOULD BE KEPT OUT OF REACH OF CHILDREN SINCE ACCIDENTAL OVERDOSAGE MAY RESULT IN SEVERE, EVEN FATAL, RESPIRATORY DEPRESSION.
MOTOFEN® may produce drowsiness or dizziness. The patient should be cautioned regarding activities requiring mental alertness, such as driving or operating dangerous machinery.
Drug Interactions
Since the chemical structure of difenoxin hydrochloride is similar to meperidine hydrochloride, the concurrent use of MOTOFEN® with monoamine oxidase inhibitors may, in theory, precipitate a hypertensive crisis.
MOTOFEN® may potentiate the action of barbiturates, tranquilizers, narcotics, and alcohol. When these medications are used concomitantly with MOTOFEN®, the patient should be closely monitored.
Diphenoxylate hydrochloride, from which the principal active metabolite difenoxin is derived, was found to inhibit the hepatic microsomal enzyme system at a dose of 2 mg/kg/day in studies conducted with male rats. Therefore, difenoxin has the potential to prolong the biological half-lives of drugs for which the rate of elimination is dependent on the microsomal drug metabolizing enzyme system.Carcinogenesis, Mutagensis, Impairment of Fertility
No evidence of carcinogenesis was found in a long-term study of difenoxin hydrochloride/atropine in the rat. In this 104 week study, rats received dietary doses of 0, 1.25, 2.5, or 5 mg/kg/day difenoxin/atropine (20:1 ratio).
No experiments have been conducted to determine the mutagenic potential of MOTOFEN®. MOTOFEN® did not
significantly impair fertility in rats.
Pregnancy/Teratogenic Effects
Pregnancy Category C. Reproduction studies in rats and rabbits with doses at 31 and 61 times the human therapeutic dose respectively, on a mg/kg basis, demonstrated no evidence of teratogenesis due to MOTOFEN®.
Pregnant rats receiving oral doses of difenoxin hydrochloride/atropine 20 times the maximum human dose had an increase in delivery time as well as a significant increase in the percent of stillbirths.
Neonatal survival in rats was also reduced with most deaths occurring within four days of delivery.
There are no well controlled studies in pregnant women. MOTOFEN® should be used during pregnancy only if the potential benefit justifies the potential risk of the fetus.
-
ADVERSE REACTIONS
In view of the small amount of atropine present (0.025 mg/tablet), such effects such as dryness of the skin and mucous membranes, flushing, hyperthermia, tachycardia and urinary retention are very unlikely to occur, except perhaps in children. Many of the adverse effects reported during clinical investigation of MOTOFEN® are difficult to distinguish from symptoms associated with the diarrheal syndrome. However, the following events were reported at the stated frequencies:
Gastrointestinal: Nausea, 1 in 15 patients; vomiting, 1 in 30 patients; dry mouth, 1 in 30 patients; epigastric distress, 1 in 100 patients; and constipation, 1 in 300 patients.
Central Nervous System: Dizziness and light-headedness, 1 in 20 patients; drowsiness, 1 in 25 patients; and headache, 1 in 40 patients; tiredness, nervousness, insomnia and confusion ranged from 1 in 200 to 1 in 600 patients.
Other less frequent reactions: Burning eyes and blurred vision occurred in a few cases.
The following adverse reactions have been reported in patients receiving chemically-related drugs: numbness of extremities, euphoria, depression, sedation, anaphylaxis, angioneurotic edema, urticaria, swelling of the gums, pruritus, toxic megacolon, paralytic ileus, pancreatitis, and anorexia.THIS MEDICATION SHOULD BE KEPT IN A CHILD-RESISTANT CONTAINER AND OUT OF THE REACH OF CHILDREN SINCE AN OVERDOSAGE MAY RESULT IN SEVERE RESPIRATORY DEPRESSION AND COMA, POSSIBLY LEADING TO PERMANENT BRAIN DAMAGE OR DEATH.
-
DRUG ABUSE AND DEPENDENCE
MOTOFEN® tablets are a Schedule IV controlled substance.
Addiction to (dependence on) difenoxin hydrochloride is theoretically possible at high dosage. Therefore, the recommended dosage should not be exceeded. Because of the structural and pharmacological similarities of difenoxin hydrochloride to drugs with a definite addiction potential, MOTOFEN® should be administered with considerable caution to patients who are receiving addicting drugs, to individuals known to be addiction prone, or to those in whom histories suggest may increase dosage on their own initiative. -
Diagnosis and Treatment
In the event of overdosage (initial signs may include dryness of the skin and mucous membranes, flushing, hyperthermia and tachycardia followed by lethargy or coma, hypotonic reflexes, nystagmus, pinpoint pupils and respiratory depression) gastric lavage, establishment of a patent airway and possibly mechanically assisted respiration are advised.
The narcotic antagonist naloxone may be used in the treatment of respiratory depression caused by narcotic analgesics of pharmacologically related compounds such as MOTOFEN® tablets. When naloxone is administered intravenously, the onset of action is generally apparent within two minutes. Naloxone may be administered subcutaneously or intramuscularly providing a slightly less rapid onset of action but a more prolonged effect.
To counteract respiratory depression caused by MOTOFEN® overdosage, the following dosage schedule for naloxone should be followed:
Adult Dosage: The usual initial adult dose of naloxone is 0.4 mg (one mL) administered intravenously. If respiratory
function does not adequately improve after the initial dose, the same IV dose may be repeated at two-to-three minute
intervals.
Children: The usual adult dose of naloxone for children is 0.01 mg/kg of body weight administered intravenously and
repeated at two-to-three minute intervals if necessary.Since the duration of action of difenoxin hydrochloride is longer than that of naloxone, improvement of respiration following administration may be followed by recurrent respiratory depression. Consequently, continuous observation is necessary until the effect of difenoxin hydrochloride on respiration (which effect may persist for many hours) has passed. Supplemental
intramuscular doses of naloxone may be utilized to produce a longer lasting effect. TREAT ALL POSSIBLE MOTOFEN® OVERDOSAGES AS SERIOUS AND MAINTAIN MEDICAL OBSERVATION FOR AT LEAST 48 HOURS, PREFERABLY UNDER CONTINUOUS HOSPITAL CARE.Although signs of overdosage and respiratory depression may not be evident soon after ingestion of difenoxin
hydrochloride, respiratory depression may occur from 12 to 30 hours later. -
DOSAGE AND ADMINISTRATION
The recommended starting dose of MOTOFEN® tablets in adults is 2 tablets then 1 tablet after each loose stool or 1 tablet every 3 to 4 hours as needed, but the total dosage during any 24-hour treatment period should not exceed 8 tablets. In the treatment of diarrhea, if clinical improvement is not observed in 48 hours, continued administration of this type of medication is not recommended. For acute diarrheas and acute exacerbations of functional diarrhea, treatment beyond 48 hours is usually not necessary.
Studies in children below the age of 12 have been inadequate to evaluate the safety and effectiveness of MOTOFEN® in this age group. MOTOFEN® is contraindicated in children under 2 years of age.
-
HOW SUPPLIED
MOTOFEN® is available as a white, dye-free, five-sided, scored tablet with “0200” on the scored side and "M" on the other side. Each tablet contains 1 mg difenoxin and 0.025 mg atropine sulfate. Supplied in bottles of 100 tablets (NDC 54766-200-10). Store at 20°-25°C (68°-77°F) [See USP Controlled Room Temperature].
- SPL UNCLASSIFIED SECTION
- 54766-200-10 Bottle Label
- 54766-200-10 Carton
-
INGREDIENTS AND APPEARANCE
MOTOFEN
difenoxin and atropine sulfate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54766-200 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPINE SULFATE (UNII: 03J5ZE7KA5) (ATROPINE - UNII:7C0697DR9I) ATROPINE SULFATE 0.025 mg DIFENOXIN HYDROCHLORIDE (UNII: VQZ63K01IW) (DIFENOXIN - UNII:3ZZ5BJ9F2Q) DIFENOXIN 1 mg Inactive Ingredients Ingredient Name Strength CALCIUM STEARATE (UNII: 776XM7047L) STARCH, CORN (UNII: O8232NY3SJ) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) Product Characteristics Color white Score 2 pieces Shape PENTAGON (5 sided) Size 7mm Flavor Imprint Code 0200;M Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54766-200-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 04/11/2017 2 NDC:54766-200-04 4 in 1 BLISTER PACK; Type 0: Not a Combination Product 04/11/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017744 04/11/2017 Labeler - Sebela Pharmaceuticals Inc. (079104574) Establishment Name Address ID/FEI Business Operations Alcami Carolinas Corporation 832395235 manufacture(54766-200)




