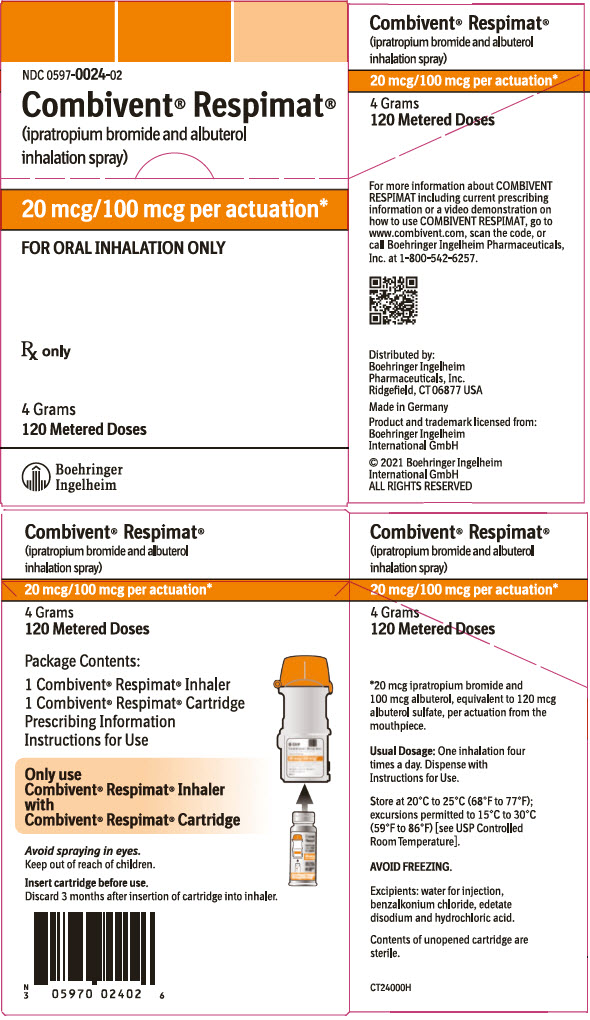
Label: COMBIVENT RESPIMAT- ipratropium bromide and albuterol spray, metered
- NDC Code(s): 0597-0024-02
- Packager: Boehringer Ingelheim Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use COMBIVENT RESPIMAT safely and effectively. See full prescribing information for COMBIVENT RESPIMAT.
COMBIVENT® RESPIMAT® (ipratropium bromide and albuterol inhalation spray), for oral inhalation use
Initial U.S. Approval: 1996INDICATIONS AND USAGE
COMBIVENT RESPIMAT Inhalation Spray is a combination of ipratropium bromide (an anticholinergic agent) and albuterol sulfate (a beta2‑adrenergic agonist) indicated for:
- Patients with chronic obstructive pulmonary disease (COPD) on a regular aerosol bronchodilator who continue to have evidence of bronchospasm and who require a second bronchodilator (1)
DOSAGE AND ADMINISTRATION
For oral inhalation only
- One inhalation four times a day, not to exceed six inhalations in 24 hours (2)
DOSAGE FORMS AND STRENGTHS
- Inhalation spray: 20 mcg ipratropium bromide (monohydrate) and 100 mcg albuterol (equivalent to 120 mcg albuterol sulfate) per actuation with the COMBIVENT RESPIMAT inhaler (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Paradoxical bronchospasm: Discontinue COMBIVENT RESPIMAT immediately and treat with alternative therapy if paradoxical bronchospasm occurs (5.1)
- Patients with cardiovascular system disorders: Use with caution because of beta-adrenergic stimulation (5.2)
- Ocular effects: Advise patients to avoid spraying into eyes and to contact a physician if blurred vision, halos, or other visual disturbances occur. Monitor patients with narrow-angle glaucoma. (5.3)
- Urinary retention: Use with caution in patients with prostatic hyperplasia or bladder-neck obstruction (5.4)
- Hypersensitivity reactions including anaphylaxis: Discontinue COMBIVENT RESPIMAT and institute alternative therapy if immediate hypersensitivity reactions such as urticaria, angioedema, rash, bronchospasm, anaphylaxis, or oropharyngeal edema occur (5.6)
- Coexisting conditions: Use with caution in patients with convulsive disorders, hyperthyroidism, or diabetes mellitus (5.7)
ADVERSE REACTIONS
Most common (≥2%) adverse reactions for COMBIVENT RESPIMAT (20/100 mcg) are upper respiratory infection, nasopharyngitis, cough, bronchitis, headache, and dyspnea (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Boehringer Ingelheim Pharmaceuticals, Inc. at (800) 542-6257 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Anticholinergics: May interact additively with concomitantly used anticholinergic medications. Avoid administration of COMBIVENT RESPIMAT with other anticholinergic-containing drugs (7.1)
- Beta-adrenergic agonists: May increase the risk of adverse cardiovascular effects. Avoid coadministration of COMBIVENT RESPIMAT and other sympathomimetic agents (7.2)
- Beta-blockers: Inhibit the effect of albuterol. Consider alternative therapy in patients with hyperreactive airways (7.3)
- Diuretics: Electrocardiographic changes and/or hypokalemia associated with diuretics may worsen with concomitant use of beta-agonists. Consider monitoring potassium levels. (7.4)
- Monoamine oxidase inhibitors (MAOs) or tricyclic antidepressants: May potentiate effect of albuterol on the vascular system. Consider alternative therapy in patients taking MAOs or tricyclic antidepressants. (7.5)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Paradoxical Bronchospasm
5.2 Cardiovascular Effects
5.3 Ocular Effects
5.4 Urinary Retention
5.5 Do Not Exceed Recommended Dose
5.6 Hypersensitivity Reactions Including Anaphylaxis
5.7 Coexisting Conditions
5.8 Hypokalemia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Anticholinergic Agents
7.2 Beta‑adrenergic Agonists
7.3 Beta-receptor Blocking Agents
7.4 Diuretics
7.5 Monoamine Oxidase Inhibitors or Tricyclic Antidepressants
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
COMBIVENT RESPIMAT is a combination of ipratropium bromide (an anticholinergic agent) and albuterol sulfate (a beta2-adrenergic agonist) indicated for use in patients with chronic obstructive pulmonary disease (COPD) on a regular aerosol bronchodilator who continue to have evidence of bronchospasm and who require a second bronchodilator.
-
2 DOSAGE AND ADMINISTRATION
The recommended dose of COMBIVENT RESPIMAT is one inhalation four times a day. Patients may take additional inhalations as required; however, the total number of inhalations should not exceed six in 24 hours.
Prior to first use, the COMBIVENT RESPIMAT cartridge is inserted into the COMBIVENT RESPIMAT inhaler and the unit is primed. When using the unit for the first time, patients are to actuate the inhaler toward the ground until an aerosol cloud is visible and then repeat the process three more times. The unit is then considered primed and ready for use. If not used for more than 3 days, patients are to actuate the inhaler once to prepare the inhaler for use. If not used for more than 21 days, patients are to actuate the inhaler until an aerosol cloud is visible and then repeat the process three more times to prepare the inhaler for use [see Patient Counseling Information (17)].
Safety and efficacy of additional doses of COMBIVENT RESPIMAT beyond six inhalations/24 hours have not been studied. Also, safety and efficacy of extra doses of ipratropium or albuterol in addition to the recommended doses of COMBIVENT RESPIMAT have not been studied.
-
3 DOSAGE FORMS AND STRENGTHS
COMBIVENT RESPIMAT consists of a COMBIVENT RESPIMAT inhaler and an aluminum cylinder (COMBIVENT RESPIMAT cartridge) containing a combination of ipratropium bromide (as the monohydrate) and albuterol sulfate. The COMBIVENT RESPIMAT cartridge is only intended for use with the COMBIVENT RESPIMAT inhaler.
Each actuation from the COMBIVENT RESPIMAT inhaler delivers 20 mcg ipratropium bromide (monohydrate) and 100 mcg albuterol (equivalent to 120 mcg albuterol sulfate) from the mouthpiece.
-
4 CONTRAINDICATIONS
COMBIVENT RESPIMAT is contraindicated in the following conditions [see Warnings and Precautions (5.6)]:
- Hypersensitivity to any of the ingredients in COMBIVENT RESPIMAT
- Hypersensitivity to atropine or any of its derivatives
-
5 WARNINGS AND PRECAUTIONS
5.1 Paradoxical Bronchospasm
COMBIVENT RESPIMAT can produce paradoxical bronchospasm that can be life-threatening. If it occurs, therapy with COMBIVENT RESPIMAT should be discontinued immediately and alternative therapy instituted.
5.2 Cardiovascular Effects
The albuterol sulfate contained in COMBIVENT RESPIMAT, like other beta‑adrenergic agonists, can produce a clinically significant cardiovascular effect in some patients, as measured by pulse rate, blood pressure, and/or symptoms. If these symptoms occur, COMBIVENT RESPIMAT may need to be discontinued. There is some evidence from postmarketing data and published literature of rare occurrences of myocardial ischemia associated with albuterol. In addition, beta‑adrenergic agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. Therefore, COMBIVENT RESPIMAT should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension [see Drug Interactions (7.2)].
5.3 Ocular Effects
Ipratropium bromide, a component of COMBIVENT RESPIMAT, is an anticholinergic and may increase intraocular pressure. This may result in precipitation or worsening of narrow-angle glaucoma. Therefore, COMBIVENT RESPIMAT should be used with caution in patients with narrow-angle glaucoma [see Drug Interactions (7.1)].
Patients should avoid spraying COMBIVENT RESPIMAT into the eyes. If a patient sprays COMBIVENT RESPIMAT into their eyes they may cause acute eye pain or discomfort, temporary blurring of vision, mydriasis, visual halos, or colored images in association with red eyes from conjunctival or corneal congestion. Advise patients to consult their physician immediately if any of these symptoms develop while using COMBIVENT RESPIMAT.
5.4 Urinary Retention
Ipratropium bromide, a component of COMBIVENT RESPIMAT, is an anticholinergic and may cause urinary retention. Therefore, caution is advised when administering this medication to patients with prostatic hyperplasia or bladder-neck obstruction [see Drug Interactions (7.1)].
5.5 Do Not Exceed Recommended Dose
Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs in patients with asthma. The exact cause of death is unknown, but cardiac arrest following an unexpected development of a severe acute asthmatic crisis and subsequent hypoxia is suspected [see Drug Interactions (7.2)].
5.6 Hypersensitivity Reactions Including Anaphylaxis
Hypersensitivity reactions including urticaria, angioedema, rash, bronchospasm, anaphylaxis, and oropharyngeal edema may occur after administration of ipratropium bromide or albuterol sulfate. In clinical trials and postmarketing experience with ipratropium containing products, hypersensitivity reactions such as skin rash, pruritus, angioedema of tongue, lips and face, urticaria (including giant urticaria), laryngospasm, and anaphylactic reactions have been reported [see Adverse Reactions (6.1, 6.2)]. If such a reaction occurs, therapy with COMBIVENT RESPIMAT should be stopped at once and alternative treatment should be considered [see Contraindications (4)].
5.7 Coexisting Conditions
COMBIVENT RESPIMAT contains albuterol sulfate, a beta2-adrenergic sympathomimetic amine and, therefore, should be used with caution in patients with convulsive disorders, hyperthyroidism, or diabetes mellitus, and in patients who are unusually responsive to sympathomimetic amines.
5.8 Hypokalemia
Beta2‑adrenergic agonists may produce significant hypokalemia in some patients (possibly through intracellular shunting) which has the potential to produce adverse cardiovascular effects. The decrease in serum potassium is usually transient, not requiring supplementation [see Drug Interactions (7.2)].
-
6 ADVERSE REACTIONS
Use of albuterol, a beta2-adrenergic agonist, may be associated with the following:
- Paradoxical bronchospasm [see Warnings and Precautions (5.1)]
- Cardiovascular effects [see Warnings and Precautions (5.2)]
- Hypersensitivity reactions including anaphylaxis [see Contraindications (4) and Warnings and Precautions (5.6)]
- Hypokalemia [see Warnings and Precautions (5.8)]
Albuterol is a component of COMBIVENT RESPIMAT.
Use of ipratropium bromide, an anticholinergic, may result in the following:
- Ocular effects [see Warnings and Precautions (5.3)]
- Urinary retention [see Warnings and Precautions (5.4)]
Ipratropium bromide is a component of COMBIVENT RESPIMAT.
6.1 Clinical Trials Experience
COMBIVENT RESPIMAT 12-Week Clinical Trials
The safety data described in Table 1 below are derived from one 12-week, randomized, multicenter, double-blind, double-dummy, parallel-group trial that compared COMBIVENT RESPIMAT (20/100 mcg), CFC-propelled COMBIVENT Inhalation Aerosol (36/206 mcg), and ipratropium bromide delivered by the RESPIMAT inhaler (20 mcg) administered four times a day in 1460 adult COPD patients (955 males and 505 females) 40 years of age and older. Of these patients, 486 were treated with COMBIVENT RESPIMAT. The COMBIVENT RESPIMAT group was composed of mostly Caucasian (88.5%) patients with a mean age of 63.8 years, and a mean percent predicted FEV1 at screening of 41.5%. Patients with narrow-angle glaucoma, symptomatic prostatic hypertrophy or bladder-neck obstruction were excluded from the trial.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Table 1 shows all adverse reactions that occurred with a frequency of ≥2% in the COMBIVENT RESPIMAT treatment group in the 12-week COPD trial. The frequency of the corresponding adverse reactions in the CFC-propelled COMBIVENT Inhalation Aerosol and ipratropium bromide delivered by the RESPIMAT inhaler groups is included for comparison. The rates are derived from all reported adverse reactions of that type not present at baseline, whether considered drug-related or not by the clinical investigator.
Table 1 Adverse Reactions in ≥2% of Patients in the COMBIVENT RESPIMAT Group in a 12-Week COPD Clinical Trial Body System (Event) 12-Week
Ipratropium-Controlled TrialCOMBIVENT RESPIMAT
(20/100 mcg)
CFC-propelled COMBIVENT Inhalation Aerosol
(36/206 mcg)Ipratropium bromide by the RESPIMAT Inhaler
(20 mcg)[n=486] [n=491] [n=483] Patients with any adverse reaction 46 52 45 Respiratory, thoracic, and mediastinal disorders Cough
Dyspnea
3
22
22
3Nervous system disorders
Headache 3
2
3
Infections and infestations Bronchitis
Nasopharyngitis
Upper Respiratory infection3
4
33
3
41
4
3Adverse reactions that occurred in <2% in the COMBIVENT RESPIMAT (20/100 mcg) group observed in this 12-week trial include: Vascular disorders: hypertension; Nervous system disorders: dizziness and tremor; Musculoskeletal and connective tissue disorder: muscle spasms and myalgia; Gastrointestinal disorders: diarrhea, nausea, dry mouth, constipation, and vomiting; General disorders and administration site conditions: asthenia, influenza-like illness, and chest discomfort; Eye disorders: eye pain; Metabolism and nutritional disorders: hypokalemia; Cardiac disorders: palpitations and tachycardia; Skin and subcutaneous tissue disorders: pruritus and rash; Respiratory, thoracic, and mediastinal disorders; pharyngolaryngeal pain and wheezing.
A separate 12-week trial evaluated a higher than approved dose of COMBIVENT RESPIMAT in 1118 COPD patients. Patients were randomized to COMBIVENT RESPIMAT (40/200 mcg) (n=345), CFC-propelled COMBIVENT Inhalation Aerosol (36/206 mcg) (n=180), ipratropium delivered by the RESPIMAT (40 mcg) (n=252) or placebo (n=341). The overall incidence and nature of adverse reactions observed were similar to the adverse reactions seen with COMBIVENT RESPIMAT 20/100 mcg.
COMBIVENT RESPIMAT Long-Term (48-week) Safety Trial
Long-term chronic use safety data for COMBIVENT RESPIMAT were obtained from one 48-week, randomized, multicenter, open-label, parallel-group trial that compared COMBIVENT RESPIMAT (20/100 mcg), CFC-propelled COMBIVENT Inhalation Aerosol (36/206 mcg), and the free combination of ipratropium bromide (34 mcg) and albuterol (180 mcg) HFA inhalation aerosols administered 4 times a day in 465 adult COPD patients (273 males and 192 females) 40 years of age and older. Of these patients, 157 were treated with COMBIVENT RESPIMAT. The COMBIVENT RESPIMAT group was composed of mostly Caucasian (93.5%) patients with a mean age of 62.9 years, and a mean percent predicted FEV1 at screening of 47.0%. An evaluation of the safety data from the trial revealed that most adverse reactions were similar in type and rate between treatment groups. However, cough occurred more frequently in patients enrolled in the COMBIVENT RESPIMAT group (7.0%) compared to those in the CFC-propelled COMBIVENT Inhalation Aerosol (2.6%) or the free combination of ipratropium bromide and albuterol HFA inhalation aerosols (3.9%) groups.
In addition to the adverse reactions reported in the controlled clinical trial with COMBIVENT RESPIMAT, adverse reaction information concerning CFC-propelled COMBIVENT Inhalation Aerosol is derived from two 12-week controlled clinical trials (N=358 for CFC-propelled COMBIVENT Inhalation Aerosol). Adverse reactions reported in ≥2% of patients in the CFC-propelled COMBIVENT Inhalation Aerosol treatment group include: bronchitis, upper respiratory tract infection, headache, dyspnea, cough, pain, respiratory disorder, sinusitis, pharyngitis, and nausea. Adverse reactions reported in <2% of patients in the CFC-propelled COMBIVENT Inhalation Aerosol treatment group include: edema, fatigue, hypertension, dizziness, nervousness, tremor, dysphonia, insomnia, diarrhea, dry mouth, dyspepsia, vomiting, arrhythmia, palpitation, tachycardia, arthralgia, angina, increased sputum, taste perversion, urinary tract infection, dysuria, dry throat, and bronchospasm.
6.2 Postmarketing Experience
In addition to the adverse reactions reported during clinical trials, the following adverse reactions have been identified during post-approval use of CFC-propelled COMBIVENT Inhalation Aerosol. Since CFC-propelled COMBIVENT Inhalation Aerosol and COMBIVENT RESPIMAT contain the same active ingredients, one should take into account the fact that the adverse reactions seen with CFC-propelled COMBIVENT Inhalation Aerosol could also occur with COMBIVENT RESPIMAT. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Eye disorders: glaucoma, blurred vision, mydriasis, conjunctival hyperemia, halo vision, accommodation disorder, ocular irritation, and corneal edema
Gastrointestinal disorders: gastrointestinal motility disorder, drying of secretions, stomatitis, and mouth edema
Immune system disorders: hypersensitivity
Investigations: intraocular pressure increased, blood pressure diastolic decreased, and blood pressure systolic increased
Musculoskeletal and connective tissue disorders: muscular weakness
Psychiatric disorders: CNS stimulation, mental disorder
Respiratory, thoracic, and mediastinal disorders: throat irritation, paradoxical bronchospasm, wheezing, nasal congestion, and pharyngeal edema
Skin and subcutaneous tissue disorders: angioedema, hyperhidrosis, and skin reaction
Urinary disorders: urinary retention
Cardiac disorders: myocardial ischemiaAllergic-type reactions such as skin reactions including rash, pruritus, and urticaria (including giant urticaria), angioedema including that of tongue, lips and face, laryngospasm, and anaphylactic reaction have also been reported with CFC-propelled COMBIVENT Inhalation Aerosol, with positive re-challenge in some cases [see Warnings and Precautions (5.6)].
In a 5-year placebo-controlled trial, hospitalizations for supraventricular tachycardia and/or atrial fibrillation occurred with an incidence rate of 0.5% in COPD patients receiving CFC-propelled ATROVENT® (ipratropium bromide) Inhalation Aerosol.
Metabolic acidosis has been reported with use of albuterol-containing products.
-
7 DRUG INTERACTIONS
COMBIVENT RESPIMAT has been used concomitantly with other drugs, including beta-adrenergic bronchodilators, methylxanthines, and oral and inhaled steroids, commonly used in the treatment of chronic obstructive pulmonary disease. There are no formal studies fully evaluating the interaction effects of COMBIVENT RESPIMAT and these drugs with respect to safety and effectiveness.
7.1 Anticholinergic Agents
There is the potential for an additive interaction with concomitantly used anticholinergic medications. Therefore, avoid coadministration of COMBIVENT RESPIMAT with other anticholinergic-containing drugs as this may lead to an increase in anticholinergic adverse effects [see Warnings and Precautions (5.3, 5.4)].
7.2 Beta‑adrenergic Agonists
Caution is advised in the coadministration of COMBIVENT RESPIMAT and other sympathomimetic agents due to the increased risk of adverse cardiovascular effects [see Warnings and Precautions (5.2, 5.5)].
7.3 Beta-receptor Blocking Agents
Beta-receptor blocking agents and albuterol inhibit the effect of each other. Beta-receptor blocking agents should be used with caution in patients with hyperreactive airways.
7.4 Diuretics
The ECG changes and/or hypokalemia which may result from the administration of non‑potassium sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta2-agonists, especially when the recommended dose of the beta2-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of beta-agonist‑containing drugs, such as COMBIVENT RESPIMAT, with non‑potassium sparing diuretics. Consider monitoring potassium levels.
7.5 Monoamine Oxidase Inhibitors or Tricyclic Antidepressants
COMBIVENT RESPIMAT should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants or within 2 weeks of discontinuation of such agents because the action of albuterol on the cardiovascular system may be potentiated. Consider alternative therapy in patients taking MAOs or tricyclic antidepressants [see Warnings and Precautions (5.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no randomized clinical studies of COMBIVENT RESPIMAT, or its individual components, ipratropium bromide and albuterol sulfate, in pregnant women. Ipratropium is negligibly absorbed systemically following oral inhalation; therefore, maternal use is not expected to result in fetal exposure to the drug [see Clinical Pharmacology (12.3)]. Published literature, including cohort studies, case control studies and case series, over several decades have not identified a drug associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes with ipratropium bromide. Available data from published epidemiological studies and postmarketing case reports of pregnancy outcomes following inhaled albuterol use do not consistently demonstrate a risk of major birth defects or, miscarriage. There are clinical considerations with the use of COMBIVENT RESPIMAT in pregnant women [see Clinical Considerations]. Animal reproduction studies have not been conducted with COMBIVENT RESPIMAT, however, animal studies are available with its individual components, ipratropium bromide and albuterol sulfate.
Based on oral reproduction studies, no evidence of structural alterations was observed when ipratropium bromide was administered to pregnant mice, rats, and rabbits during organogenesis at doses approximately 340, 68,000 and 17,000 times, respectively, the maximum recommended human daily inhalation dose (MRHDID) in adults on a mg/m2 basis.
When albuterol was administered to pregnant mice during organogenesis there was evidence of cleft palate at doses approximately equivalent to the maximum recommended human daily inhalation dose (MRHDID) [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Consideration
Labor or Delivery
Because of the potential for beta-agonist interference with uterine contractility, use of COMBIVENT RESPIMAT for the treatment of COPD during labor should be restricted to those patients in whom the benefits clearly outweigh the risk. Serious adverse reactions, including pulmonary edema, have been reported during or following treatment of premature labor with beta2-agonists, including albuterol.
Data
Animal Data
Ipratropium bromide
In animal reproduction studies, oral and inhalation administration of ipratropium bromide to pregnant mice, rats and rabbits during the period of organogenesis did not show evidence of fetal structural alterations. The ipratropium dose in oral studies in mice, rats, and rabbits was up to approximately 340, 68,000 and 17,000 times, respectively, the maximum recommended human daily inhalation dose (MRHDID) in adults (on a mg/m2 basis at maternal doses in each species of 10, 1000 and 125 mg/kg/day, respectively). The ipratropium dose in inhalation studies in rats and rabbits was up to approximately 100 and 240 times, respectively, the MRHDID in adults (on a mg/m2 basis at maternal doses of 1.5 and 1.8 mg/kg/day, respectively). Embryotoxicity was observed as increased resorption in rats at oral doses approximately 6100 times MRHDID in adults (on a mg/m2 basis at maternal doses of 90 mg/kg/day and above). This effect is not considered relevant to human use due to the large doses at which it was observed and the difference in route of administration.
Albuterol
In a mouse reproduction study, subcutaneously administered albuterol sulfate produced cleft palate formation in 5 of 111 (4.5%) fetuses at a dose approximately equivalent to the MRHDID in adults (on a mg/m2 basis at a maternal dose of 0.25 mg/kg/day) and in 10 of 108 (9.3%) fetuses at approximately 14 times the MRHDID in adults (on a mg/m2 basis at a maternal dose of 2.5 mg/kg/day). Similar effects were not observed at approximately less than MRHDID in adults (on a mg/m2 basis at a maternal dose of 0.025 mg/kg/day). Cleft palate also occurred in 22 of 72 (30.5%) fetuses from females treated with 2.5 mg/kg/day isoproterenol (positive control).
In a rabbit reproductive study, orally administered albuterol sulfate induced cranioschisis in 7 of 19 (37%) fetuses at approximately 1100 times the MRHDID in adults (on a mg/m2 basis at a maternal dose of 50 mg/kg/day).
In a rat reproduction study, an albuterol sulfate/HFA-134a formulation administered by inhalation did not produce any teratogenic effects at exposures approximately 80 times the MRHDID (on a mg/m2 basis at a maternal dose of 10.5 mg/kg). A study in which pregnant rats were dosed with radiolabeled albuterol sulfate demonstrated that drug-related material is transferred from the maternal circulation to the fetus.
8.2 Lactation
Risk Summary
There are no available data on the presence of COMBIVENT RESPIMAT, or its components, ipratropium bromide or albuterol, in human milk, the effects on the breastfed infant, or the effects on milk production. Although lipid-insoluble quaternary cations pass into breast milk, ipratropium concentrations in plasma after inhaled therapeutic doses are low. Similarly, plasma levels of albuterol after inhaled therapeutic doses are low in humans. Therefore, ipratropium and albuterol concentrations in human breast milk are likely to be correspondingly low [see Clinical Pharmacology (12.3)]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for albuterol and any potential adverse effects on the breastfed child from albuterol or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of COMBIVENT RESPIMAT in pediatric patients have not been established. COMBIVENT RESPIMAT is indicated for use in patients with COPD on a regular aerosol bronchodilator who continue to have evidence of bronchospasm and who require a second bronchodilator. This disease does not normally occur in children.
8.5 Geriatric Use
In the 12-week trial in COPD, 48% of COMBIVENT RESPIMAT clinical trial patients were 65 years of age or over. In general, there were no marked differences between the proportion of patients with adverse reactions for the COMBIVENT RESPIMAT and CFC-propelled COMBIVENT Inhalation Aerosol treated patients. Cardiac and lower respiratory disorders occurred less frequently in the patients under the age of 65 and were balanced across treatment groups.
No overall differences in effectiveness were observed among treatment groups. Based on available data, no adjustment of COMBIVENT RESPIMAT dosage in geriatric patients is warranted.
-
10 OVERDOSAGE
The effects of overdosage are expected to be related primarily to albuterol sulfate. Acute overdosage with ipratropium bromide by inhalation is unlikely since ipratropium bromide is not well absorbed systemically after inhalation or oral administration. Manifestations of overdosage with albuterol may include anginal pain, hypertension, hypokalemia, tachycardia with rates up to 200 beats per minute, metabolic acidosis, and exaggeration of the pharmacologic effects listed in the Adverse Reactions section [see Adverse Reactions (6)]. As with all beta2-adrenergic agonist aerosol medications, cardiac arrest and even death may be associated with abuse.
Treatment of overdosage consists of discontinuation of COMBIVENT RESPIMAT together with institution of appropriate medical and supportive therapy. Dialysis is not appropriate treatment for overdosage of albuterol as an inhalation aerosol; the judicious use of a cardiovascular beta‑receptor blocker, such as metoprolol tartrate, may be indicated.
-
11 DESCRIPTION
COMBIVENT RESPIMAT is a combination of ipratropium bromide (as the monohydrate) and albuterol sulfate.
Ipratropium bromide is an anticholinergic bronchodilator chemically described as 8-azoniabicyclo[3.2.1] octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-, bromide monohydrate, (3-endo, 8-syn)-: a synthetic quaternary ammonium compound chemically related to atropine. Ipratropium bromide is a white to off-white crystalline substance, freely soluble in water and methanol, sparingly soluble in ethanol, and insoluble in lipophilic solvents such as ether, chloroform, and fluorocarbons.
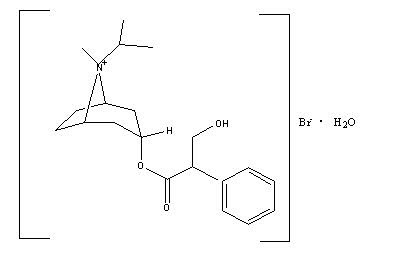
C20H30BrNO3•H2O ipratropium bromide Mol. Wt. 430.4
Albuterol sulfate, chemically known as (1,3-benzenedimethanol, α'-[[(1,1dimethylethyl) amino] methyl]-4-hydroxy, sulfate (2:1)(salt), (±)- is a relatively selective beta2-adrenergic bronchodilator. Albuterol is the official generic name in the United States. The World Health Organization recommended name for the drug is salbutamol. Albuterol sulfate is a white to off-white crystalline powder, freely soluble in water and slightly soluble in alcohol, chloroform, and ether.
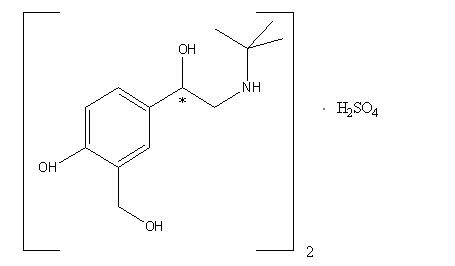
(C13H21NO3)2•H2SO4 albuterol sulfate Mol. Wt. 576.7
The drug product, COMBIVENT RESPIMAT, is composed of a sterile, aqueous solution of ipratropium bromide and albuterol sulfate filled into a 4.5 mL plastic container crimped into an aluminum cylinder (COMBIVENT RESPIMAT cartridge) for use with the COMBIVENT RESPIMAT inhaler. Excipients include water for injection, benzalkonium chloride, edetate disodium, and hydrochloric acid. The COMBIVENT RESPIMAT cartridge is only intended for use with the COMBIVENT RESPIMAT inhaler. The COMBIVENT RESPIMAT inhaler is a hand held, pocket sized oral inhalation device that uses mechanical energy to generate a slow moving aerosol cloud of medication from a metered volume of the drug solution. The COMBIVENT RESPIMAT inhaler has an orange-colored cap.
When used with the COMBIVENT RESPIMAT inhaler, each cartridge containing 4 grams of a sterile aqueous solution delivers the labeled number of metered actuations after preparation for use. Each actuation from the COMBIVENT RESPIMAT inhaler delivers 20 mcg ipratropium bromide (monohydrate) and 100 mcg albuterol (equivalent to 120 mcg albuterol sulfate) in 11.4 mcL of solution from the mouthpiece. As with all inhaled drugs, the actual amount of drug delivered to the lung may depend on patient factors, such as the coordination between the actuation of the inhaler and inspiration through the delivery system. The duration of inspiration should be at least as long as the spray duration (1.5 seconds).
Prior to first use, the COMBIVENT RESPIMAT cartridge is inserted into the COMBIVENT RESPIMAT inhaler and the unit is primed. When using the unit for the first time, patients are to actuate the inhaler toward the ground until an aerosol cloud is visible and then repeat the process three more times. The unit is then considered primed and ready for use. If not used for more than 3 days, patients are to actuate the inhaler once to prepare the inhaler for use. If not used for more than 21 days, patients are to actuate the inhaler until an aerosol cloud is visible and then repeat the process three more times to prepare the inhaler for use [see Patient Counseling Information (17)].
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
COMBIVENT RESPIMAT: COMBIVENT RESPIMAT is a combination of the anticholinergic ipratropium bromide and the beta2-adrenergic agonist albuterol sulfate. The mechanisms of action described below for the individual components apply to COMBIVENT RESPIMAT. The two classes of medications (an anticholinergic and a beta2-adrenergic agonist) are both bronchodilators. Simultaneous administration of both an anticholinergic (ipratropium bromide) and a beta2-sympathomimetic (albuterol sulfate) is designed to produce a greater bronchodilator effect than when either drug is utilized alone at its recommended dosage. The efficacy of COMBIVENT RESPIMAT is likely to be due to a local effect on the muscarinic and beta2-adrenergic receptors in the lung.
Ipratropium bromide
Ipratropium bromide is an anticholinergic (parasympatholytic) agent which, based on animal studies, appears to inhibit vagally mediated reflexes by antagonizing the action of acetylcholine, the transmitter agent released at the neuromuscular junctions in the lung. Anticholinergics prevent the increases in intracellular concentration of Ca++ which is caused by interaction of acetylcholine with the muscarinic receptors on bronchial smooth muscle.
Albuterol sulfate
In vitro studies and in vivo pharmacology studies have demonstrated that albuterol has a preferential effect on beta2-adrenergic receptors compared with isoproterenol. While it is recognized that beta2-adrenergic receptors are the predominant receptors on bronchial smooth muscle, recent data indicate that there is a population of beta2-receptors in the human heart which comprise between 10% and 50% of cardiac beta-adrenergic receptors. The precise function of these receptors, however, is not yet established [see Warnings and Precautions (5.2)].
Activation of beta2-adrenergic receptors on airway smooth muscle leads to the activation of adenylyl cyclase and to an increase in the intracellular concentration of cyclic-3',5'-adenosine monophosphate (cyclic AMP). This increase of cyclic AMP leads to the activation of protein kinase, which inhibits the phosphorylation of myosin and lowers intracellular ionic calcium concentrations, resulting in relaxation. Albuterol relaxes the smooth muscles of all airways, from the trachea to the terminal bronchioles. Albuterol acts as a functional antagonist to relax the airway irrespective of the spasmogen involved, thus protecting against all bronchoconstrictor challenges. Increased cyclic AMP concentrations are also associated with the inhibition of release of mediators from mast cells in the airway.
Albuterol has been shown in most clinical trials to have more bronchial smooth muscle relaxation effect than isoproterenol at comparable doses while producing fewer cardiovascular effects. However, all beta‑adrenergic agonist drugs, including albuterol sulfate, can produce a significant cardiovascular effect in some patients [see Warnings and Precautions (5.2)].
12.2 Pharmacodynamics
Ipratropium bromide
Cardiovascular effects
At recommended doses, ipratropium bromide does not produce clinically significant changes in pulse rate or blood pressure.Ocular effects
In studies without a positive control, ipratropium bromide did not alter pupil size, accommodation, or visual acuity.Mucociliary clearance and respiratory secretions
Controlled clinical studies have demonstrated that ipratropium bromide does not alter either mucociliary clearance or the volume or viscosity of respiratory secretions.Albuterol sulfate
Cardiovascular effects
Controlled clinical trials and other clinical experience have shown that inhaled albuterol, like other beta-adrenergic agonist drugs, can produce a significant cardiovascular effect in some patients, as measured by pulse rate, blood pressure, symptoms, and/or electrocardiographic changes.12.3 Pharmacokinetics
Ipratropium bromide
Ipratropium bromide is a quaternary amine and hence, it is not readily absorbed into the systemic circulation either from the surface of the lung or from the gastrointestinal tract as confirmed by blood level and renal excretion studies.
The half-life of elimination is about 2 hours after inhalation or intravenous administration. Ipratropium bromide is minimally bound (0% to 9% in vitro) to plasma albumin and α1-acid glycoprotein. It is partially metabolized to inactive ester hydrolysis products. Following intravenous administration, approximately one-half of the dose is excreted unchanged in the urine.
Albuterol sulfate
Albuterol is longer acting than isoproterenol in most patients because it is not a substrate for the cellular uptake processes for catecholamines, nor for metabolism by catechol-O-methyl transferase. Instead, the drug is conjugatively metabolized to albuterol 4'-O-sulfate.
Intravenous pharmacokinetics of albuterol was studied in a comparable group of 16 healthy male volunteers; the mean terminal half-life following a 30-minute infusion of 1.5 mg was 3.9 hours, with a mean clearance of 439 mL/min/1.73 m2.
COMBIVENT RESPIMAT Inhalation Spray
In a 12-week randomized, multicenter, double-blind, double-dummy parallel group trial, 108 US patients with COPD receiving either COMBIVENT RESPIMAT (20/100 mcg) or CFC-propelled COMBIVENT Inhalation Aerosol (36/206 mcg) four times daily participated in pharmacokinetic evaluations. Plasma ipratropium concentrations were low with an average peak plasma concentration of 33.5 pg/mL from COMBIVENT RESPIMAT. The majority of the study participants exhibited levels below the lower limit of quantitation (<10 pg/mL) by 4 to 6 hours following dosing. The steady state systemic exposure obtained for ipratropium bromide following COMBIVENT RESPIMAT was comparable to that of CFC-propelled COMBIVENT Inhalation Aerosol. Ipratropium plasma AUC and total amount of drug excreted unchanged in urine (Ae) ratios for COMBIVENT RESPIMAT/CFC-propelled COMBIVENT Inhalation Aerosol were 1.04 and 1.18, respectively. For albuterol the steady state systemic exposure was less from COMBIVENT RESPIMAT compared to that of CFC-propelled COMBIVENT Inhalation Aerosol. Albuterol plasma AUC and urine Ae ratios for COMBIVENT RESPIMAT/CFC-propelled COMBIVENT Inhalation Aerosol were 0.74 and 0.86, respectively.
Pharmacokinetic drug-drug interaction between ipratropium bromide and albuterol sulfate was evaluated in a crossover study in 12 healthy male volunteers who received CFC-propelled COMBIVENT Inhalation Aerosol and the two active components separately as individual treatments. Results from this study indicated that the coadministration of these two components from a single canister did not significantly alter the systemic absorption of either component, indicating lack of any pharmacokinetic interaction between these two drugs.
Specific Populations
Age
Consistent with CFC-propelled COMBIVENT Inhalation Aerosol (36/206 mcg), patients receiving COMBIVENT RESPIMAT (20/100 mcg) aged 65 years and over had higher steady state systemic exposures than patients aged under 65 years for both ipratropium (AUC = 166 vs. 105 pg•hr/mL, Cmax = 38.5 vs. 30.1 pg/mL) and albuterol (AUC = 5.44 vs. 3.27 ng•hr/mL, Cmax = 1.19 vs. 0.74 ng/mL).Gender
The AUC and Cmax values for ipratropium were 131 pg.hr/mL and 35.4 pg/mL in males and 123 pg.hr/mL and 31.7 pg/mL in females, respectively. The AUC- and Cmax-values for albuterol were 4.0 ng•hr/mL and 0.89 ng/mL in males and 4.2 ng•hr/mL and 0.93 ng/mL in females, respectively.Hepatic and Renal Impairment
The pharmacokinetics of COMBIVENT RESPIMAT or ipratropium bromide has not been studied in patients with hepatic or renal insufficiency.Drug-Drug Interactions
No specific pharmacokinetic studies were conducted to evaluate potential drug-drug interactions with other medications. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Ipratropium bromide
Two-year oral carcinogenicity studies in rats and mice have revealed no carcinogenic activity at doses up to 6 mg/kg/day (approximately 400 and 200 times the maximum recommended human daily inhalation dose of ipratropium bromide (MRHDID) in adults on a mg/m2 basis, respectively).
Results of various mutagenicity/clastogenicity studies (Ames test, mouse dominant lethal test, mouse micronucleus test, and chromosome aberration of bone marrow in Chinese hamsters) were negative.
Fertility of male or female rats at oral doses up to 50 mg/kg/day (approximately 3400 times the MRHDID in adults on a mg/m2 basis) was unaffected by ipratropium bromide administration. At an oral dose of 500 mg/kg/day (approximately 34,000 times the MRHDID in adults on a mg/m2 basis), ipratropium bromide produced a decrease in the conception rate.
Albuterol
Like other agents in its class, albuterol caused a significant dose-related increase in the incidence of benign leiomyomas of the mesovarium in a 2-year study in the rat at dietary doses of 2, 10, and 50 mg/kg/day (approximately 20, 110, and 560 times the MRHDID on a mg/m2 basis). In another study this effect was blocked by the coadministration of propranolol. The relevance of these findings to humans is not known. An 18-month study in mice at dietary doses up to 500 mg/kg/day (approximately 2800 times the MRHDID on a mg/m2 basis) and a 99-week study in hamsters at oral doses up to 50 mg/kg/day (approximately 470 times the MRHDID on a mg/m2 basis) revealed no evidence of tumorigenicity. Studies with albuterol revealed no evidence of mutagenesis.
Reproduction studies in rats with albuterol sulfate revealed no evidence of impaired fertility.
13.2 Animal Toxicology and/or Pharmacology
Preclinical: Intravenous studies in rats with albuterol sulfate have demonstrated that albuterol crosses the blood-brain barrier and reaches brain concentrations amounting to approximately 5% of the plasma concentrations. In structures outside the blood-brain barrier (pineal and pituitary glands), albuterol concentrations were found to be 100 times those in the whole brain.
Studies in laboratory animals (minipigs, rodents, and dogs) have demonstrated the occurrence of cardiac arrhythmias and sudden death (with histologic evidence of myocardial necrosis) when beta-agonists and methylxanthines were administered concurrently. The clinical significance of these findings is unknown.
-
14 CLINICAL STUDIES
The efficacy of COMBIVENT RESPIMAT (20/100 mcg) was evaluated in COPD patients in one randomized, double-blind, double-dummy parallel group trial. This was a 12-week trial in a total of 1460 adult patients (955 males and 505 females) conducted to demonstrate the efficacy and safety of COMBIVENT RESPIMAT (20/100 mcg) in COPD. All patients were required to have a clinical diagnosis of COPD, be at least 40 years of age or older, to have an FEV1 of less than or equal to 65% predicted and an FEV1/FVC ratio of less than or equal to 0.7 at screening, and a smoking history of greater than 10 pack-years prior to entering the trial. Patients with narrow-angle glaucoma, symptomatic prostatic hypertrophy, or bladder neck obstruction were excluded from the trial. The majority of the patients (89%) were Caucasian, had a mean age of 64 years, a mean percent predicted pre-bronchodilator FEV1 of 41% and FEV1/FVC ratio of 0.45. The patients were randomized to one of the following active treatments COMBIVENT RESPIMAT (20/100 mcg) (n=486), CFC-propelled COMBIVENT Inhalation Aerosol (36/206 mcg) (n=491), and ipratropium bromide delivered by the RESPIMAT (20 mcg) (n=483) administered four times a day. Data from 1424 patients were used in the efficacy analyses.
There were three primary efficacy variables: (i) Mean FEV1 over 0 to 6 hours post-dose defined as the AUC of the change from test-day baseline in FEV1 over 0 to 6 hours post-dose divided by 6 hours (FEV1 AUC0-6h); (ii) Mean FEV1 over 0 to 4 hours post-dose defined as the AUC of the change from test-day baseline in FEV1 over 0 to 4 hours post-dose divided by 4 hours (FEV1 AUC0-4h), and (iii) Mean FEV1 over 4 to 6 hours post-dose defined as the AUC of the change from test-day baseline in FEV1 over 4 to 6 hours post-dose divided by 2 hours (FEV1 AUC4-6h). Test-day baseline was the FEV1 recorded prior to inhaling the dose of randomized treatment on test day.
The three primary efficacy comparisons were: (i) Non-inferiority of COMBIVENT RESPIMAT (20/100 mcg) to CFC-propelled COMBIVENT Inhalation Aerosol (36/206 mcg) for the FEV1 AUC0-6h on Test Day 85; (ii) Superiority of COMBIVENT RESPIMAT (20/100 mcg) to ipratropium RESPIMAT (20 mcg) for the FEV1 AUC0-4h on Test Day 85, to demonstrate the contribution of albuterol in the combination product, and (iii) Non-inferiority of COMBIVENT RESPIMAT (20/100 mcg) in comparison to ipratropium RESPIMAT (20 mcg) for FEV1 AUC4-6h on Test Day 85, to demonstrate the contribution of ipratropium in the combination product. Non-inferiority was declared if the lower bound of the 95% confidence interval for the point estimate for the difference of COMBIVENT RESPIMAT minus the comparator was more than -50 mL.
COMBIVENT RESPIMAT (20/100 mcg) was shown to be non-inferior to CFC-propelled COMBIVENT Inhalation Aerosol (36/206 mcg) in terms of mean FEV1 AUC0-6h. The LS mean (mL) (95% CI) of the treatment difference was -3 (-22, 15). The FEV1 AUC0-4h for COMBIVENT RESPIMAT (20/100 mcg), was superior to that of ipratropium bromide [LS mean (mL) (95% CI) of the treatment difference was 47 mL (28, 66)] and the mean FEV1 AUC4-6h for COMBIVENT RESPIMAT (20/100 mcg) was non-inferior to that of ipratropium bromide [LS mean (mL) (95% CI) of the treatment difference was -17 (-39, 5)]. The FEV1 results on Test Days 1, 29, 57, and 85 are shown in Figure 1.
In this trial, COMBIVENT RESPIMAT (20/100 mcg) was shown to be clinically comparable (statistically non-inferior) to CFC-propelled COMBIVENT Inhalation Aerosol (36/206 mcg).
Additionally, in this trial, no differences in these efficacy comparisons were identified in males and females or in patients over 65 years of age versus those under 65 years of age. There were too few African-American subjects to adequately assess differences in effects in that population.
The median time to onset of bronchodilation, defined as an FEV1 increase of 15% or greater from test-day baseline, for the COMBIVENT RESPIMAT (20/100 mcg) group occurred at 13 minutes post-dose on Day 1.
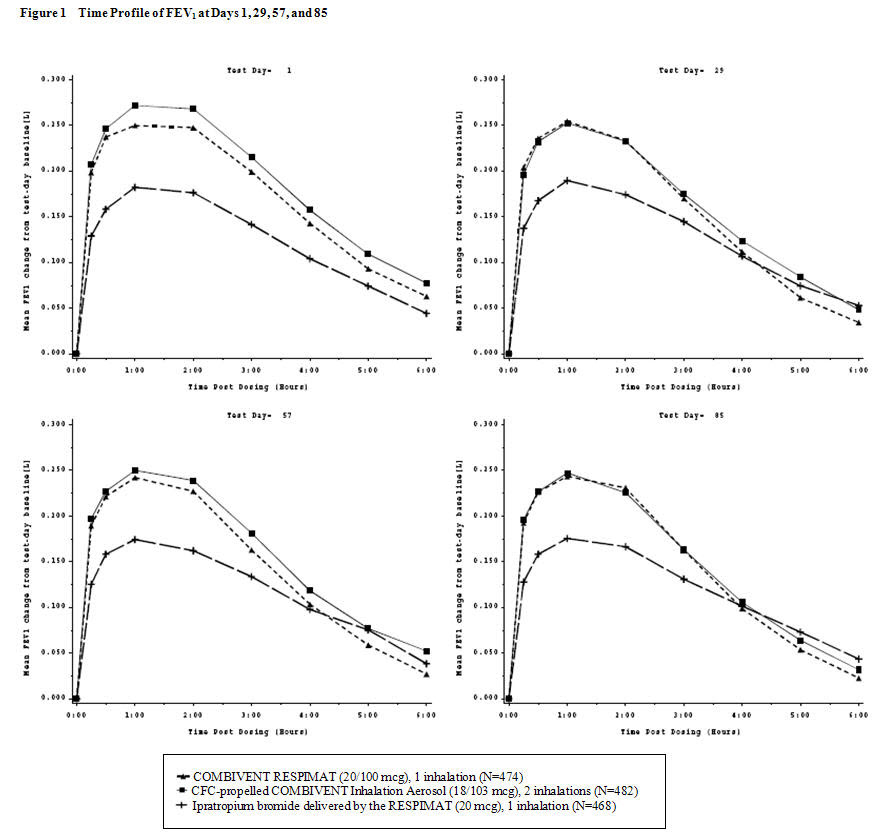
The means are adjusted for treatment baseline and investigator site. A separate ANCOVA was fitted for each time point.
The imputation method for data missing because the patient withdrew from the trial was Last Visit Carried Forward.
The imputation method for data missing at the end of test days depends on why the data were missing.A second study was conducted in 1118 COPD patients using a higher than approved dose of COMBIVENT RESPIMAT. Patients were randomized to COMBIVENT RESPIMAT (40/200 mcg) (n=345), CFC-propelled COMBIVENT Inhalation Aerosol (36/206 mcg) (n=180), ipratropium delivered by the RESPIMAT (40 mcg) (n=252) or placebo (n= 341). The study was supportive, particularly for safety [see Adverse Reactions (6.1)].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
COMBIVENT RESPIMAT Inhalation Spray is supplied in a carton containing one COMBIVENT RESPIMAT cartridge and one COMBIVENT RESPIMAT inhaler.
The COMBIVENT RESPIMAT cartridge is provided as an aluminum cylinder with a tamper protection seal on the cap. The COMBIVENT RESPIMAT cartridge is only intended for use with the COMBIVENT RESPIMAT inhaler.
The COMBIVENT RESPIMAT inhaler is a cylindrical shaped plastic inhalation device with a gray colored body and a clear base. The clear base is removed to insert the cartridge. The inhaler contains a dose indicator. The orange-colored cap and the written information on the label of the gray inhaler body indicate that it is labeled for use with the COMBIVENT RESPIMAT cartridge.
COMBIVENT RESPIMAT Inhalation Spray is available as:
COMBIVENT RESPIMAT Inhalation Spray: 120 metered actuations (NDC 0597-0024-02))The COMBIVENT RESPIMAT cartridge has a net fill weight of 4 grams and when used with the COMBIVENT RESPIMAT inhaler, is designed to deliver the labeled number of metered actuations after preparation for use. Each actuation from the COMBIVENT RESPIMAT inhaler delivers 20 mcg ipratropium bromide (monohydrate) and 100 mcg albuterol (equivalent to 120 mcg albuterol sulfate) from the mouthpiece.
When the labeled number of metered actuations has been dispensed from the inhaler, the RESPIMAT locking mechanism will be engaged and no more actuations can be dispensed.
After assembly, the COMBIVENT RESPIMAT inhaler should be discarded at the latest 3 months after first use or when the locking mechanism is engaged, whichever comes first.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Inform patients that COMBIVENT RESPIMAT can produce paradoxical bronchospasm that can be life-threatening. If paradoxical bronchospasm occurs, patients should discontinue using COMBIVENT RESPIMAT.
Caution patients to avoid spraying the aerosol into their eyes and advise that this may result in precipitation or worsening of narrow‑angle glaucoma, mydriasis, increased intraocular pressure, acute eye pain or discomfort, temporary blurring of vision, visual halos or colored images in association with red eyes from conjunctival and corneal congestion. Patients should also be advised that should any combination of these symptoms develop, they should consult their physician immediately.
Since dizziness, accommodation disorder, mydriasis, and blurred vision may occur with use of COMBIVENT RESPIMAT, patients should be cautioned about engaging in activities requiring balance and visual acuity such as driving a car or operating appliances or machinery.
Inform patients that COMBIVENT RESPIMAT may cause urinary retention and advise them to consult their physician if they experience difficulty with urination.
Adverse Effects Associated with Beta2-agonists
Inform patients of adverse effects associated with beta2-agonists, such as palpitations, chest pain, rapid heart rate, tremor, or nervousness [see Warnings and Precautions (5.2, 5.5)].
The action of COMBIVENT RESPIMAT should last 4 to 5 hours or longer. COMBIVENT RESPIMAT should not be used more frequently than recommended. Safety and efficacy of additional doses of COMBIVENT RESPIMAT beyond six inhalations in 24 hours have not been studied. Patients should be told not to increase the dose or frequency of COMBIVENT RESPIMAT without consulting a physician. Patients should be instructed that if they find that treatment with COMBIVENT RESPIMAT becomes less effective for symptomatic relief, their symptoms become worse, and/or they need to use the product more frequently than usual, medical attention should be sought immediately [see Warnings and Precautions (5.5)].
Hypersensitivity Reactions
Inform patients that hypersensitivity reactions, including urticaria, angioedema, rash, bronchospasm, anaphylaxis, and oropharyngeal edema, may occur after the administration of COMBIVENT RESPIMAT. Advise patients to immediately discontinue COMBIVENT RESPIMAT and consult a physician [see Warnings and Precautions (5.6)].
Remind patients that while taking COMBIVENT RESPIMAT, other inhaled drugs should be taken only as directed by a physician [see Drug Interactions (7.1)].
Patients who are pregnant or nursing should contact their physician about the use of COMBIVENT RESPIMAT [see Use in Specific Populations (8.1, 8.2)].
Preparation for Use and Priming
Instruct patients that priming COMBIVENT RESPIMAT is essential to ensure appropriate content of the medication in each actuation.
When using the unit for the first time, the COMBIVENT RESPIMAT cartridge is inserted into the COMBIVENT RESPIMAT inhaler and the unit is primed. COMBIVENT RESPIMAT patients are to actuate the inhaler toward the ground until an aerosol cloud is visible and then repeat the process three more times. The unit is then considered primed and ready for use. If not used for more than 3 days, patients are to actuate the inhaler once to prepare the inhaler for use. If not used for more than 21 days, patients are to actuate the inhaler until an aerosol cloud is visible and then repeat the process three more times to prepare the inhaler for use.
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USALicensed from:
Boehringer Ingelheim International GmbHRESPIMAT® is a registered trademark of and used under license from Boehringer Ingelheim International GmbH
COMBIVENT® is a registered trademark of and used under license from Boehringer Ingelheim Pharmaceuticals, Inc.
Copyright © 2021 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED -
PATIENT PACKAGE INSERT
Instructions for Use
COMBIVENT® RESPIMAT® (COM beh vent - RES peh mat)
(ipratropium bromide and albuterol inhalation spray)For Oral Inhalation Only
Do not spray COMBIVENT RESPIMAT into your eyes.Read these Instructions for Use before you start using COMBIVENT RESPIMAT and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your doctor about your medical condition or your treatment.
Use COMBIVENT RESPIMAT exactly as prescribed by your doctor. Do not change your dose or how often you use COMBIVENT RESPIMAT without talking with your doctor.
Tell your doctor about all of the medicines you take. COMBIVENT RESPIMAT may affect the way some medicines work and some other medicines may affect the way COMBIVENT RESPIMAT works. Do not use other inhaled medicines with COMBIVENT RESPIMAT without talking to your doctor.
The COMBIVENT RESPIMAT inhaler has a slow moving mist that helps you inhale the medicine.
Do not turn the clear base before inserting the cartridge.
How to store your COMBIVENT RESPIMAT inhaler
- Store COMBIVENT RESPIMAT at room temperature 68°F to 77°F (20°C to 25°C).
- Do not freeze your COMBIVENT RESPIMAT cartridge and inhaler.
- If COMBIVENT RESPIMAT has not been used for more than 3 days, release 1 puff towards the ground.
- If COMBIVENT RESPIMAT has not been used for more than 21 days, repeat steps 4 to 6 under the “Prepare for first use” until a mist is visible. Then repeat steps 4 to 6 three more times.
- Keep your COMBIVENT RESPIMAT cartridge and inhaler out of the reach of children.
How to care for your COMBIVENT RESPIMAT inhaler
Clean the mouthpiece, including the metal part inside the mouthpiece, with a damp cloth or tissue only, at least once a week. Any minor discoloration in the mouthpiece does not affect your COMBIVENT RESPIMAT inhaler.
When to get a new COMBIVENT RESPIMAT inhaler
- Your inhaler contains 120 puffs (120 doses); or if you have a sample, your inhaler contains 60 puffs (60 doses) instead.
- The dose indicator shows approximately how much medicine is left.
- When the dose indicator enters the red area of the scale you need to get a refill; there is approximately medicine for 7 days left (if you have a sample, there is approximately medicine for 3 days left).
- When the dose indicator reaches the end of the red scale, your COMBIVENT RESPIMAT is empty and automatically locks. At this point, the clear base cannot be turned any further.
- Three months after insertion of cartridge, throw away the COMBIVENT RESPIMAT even if it has not been used, or when the inhaler is locked, or when it expires, whichever comes first.
Prepare for first use
1. Remove clear base - Keep the cap closed.
- Press the safety catch while firmly pulling off the clear base with your other hand. Be careful not to touch the piercing element.
- Write the discard by date on the label (3 months from the date the cartridge is inserted).

2. Insert cartridge - Insert the narrow end of the cartridge into the inhaler.
- Place the inhaler on a firm surface and push down firmly until it clicks into place.
3. Replace clear base - Put the clear base back into place until it clicks.
- Do not remove the clear base or the cartridge after it has been put together.
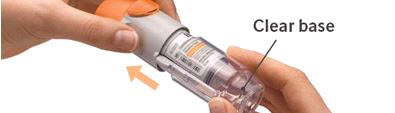
4. Turn - Keep the cap closed.
- Turn the clear base in the direction of the arrows on the label until it clicks (half a turn).

5. Open - Open the cap until it snaps fully open.

6. Press - Point the inhaler toward the ground.
- Press the dose-release button.
- Close the cap.
- If you do not see a mist, repeat steps 4 to 6 until a mist is seen.
- After a mist is seen, repeat steps 4 to 6 three more times.
- After complete preparation of your inhaler, it will be ready to deliver the number of puffs on the label.

Turn - Keep the cap closed.
- Turn the clear base in the direction of the arrows on the label until it clicks (half a turn).

Open - Open the cap until it snaps fully open.

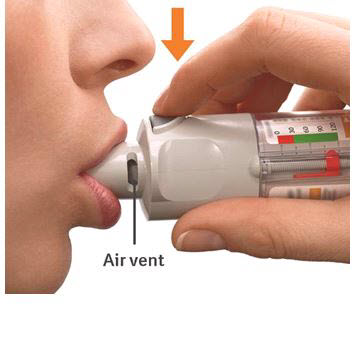
Press - Breathe out slowly and fully.
- Close your lips around the mouthpiece without covering the air vents.
- Point the inhaler to the back of your throat.
- While taking a slow, deep breath through your mouth, Press the dose-release button and continue to breathe in.
- Hold your breath for 10 seconds or for as long as comfortable.
- Close the cap until you use your inhaler again.
It is difficult to insert the cartridge deep enough:
Did you accidentally turn the clear base before inserting the cartridge? Open the cap, press the dose-release button, then insert the cartridge.
Did you insert the cartridge with the wide end first? Insert the cartridge with the narrow end first.
I cannot press the dose-release button:
Did you turn the clear base? If not, turn the clear base in a continuous movement until it clicks (half a turn).
Is the dose indicator on the COMBIVENT RESPIMAT pointing to zero? The COMBIVENT RESPIMAT inhaler is locked after 120 puffs (120 doses). If you have a sample, the COMBIVENT RESPIMAT inhaler is locked after 60 puffs (60 doses). Prepare and use your new COMBIVENT RESPIMAT inhaler.
Did you turn the clear base already? If the clear base has already been turned, follow steps “Open” and “Press” under “Daily use” to get your medicine.
Is the dose indicator on the COMBIVENT RESPIMAT pointing to zero? The COMBIVENT RESPIMAT inhaler is locked after 120 puffs (120 doses). If you have a sample, the COMBIVENT RESPIMAT inhaler is locked after 60 puffs (60 doses). Prepare and use your new COMBIVENT RESPIMAT inhaler.
The dose indicator on the COMBIVENT RESPIMAT reaches zero too soon:
Did you use COMBIVENT RESPIMAT as indicated (1 puff four times daily)? COMBIVENT RESPIMAT will deliver 120 puffs and last 30 days if used at 1 puff four times daily. If you have a sample, COMBIVENT RESPIMAT will deliver 60 puffs and last 15 days if used at 1 puff four times daily.
Did you turn the clear base before you inserted the cartridge? The dose indicator counts each turn of the clear base regardless whether a cartridge has been inserted or not.
Did you spray in the air often to check whether the COMBIVENT RESPIMAT is working? Once you have prepared COMBIVENT RESPIMAT, no test-spraying is required if used daily.
Did you insert the cartridge into a used COMBIVENT RESPIMAT? Always insert a new cartridge into a NEW COMBIVENT RESPIMAT.
My COMBIVENT RESPIMAT sprays automatically:
Was the cap open when you turned the clear base? Close the cap, then turn the clear base.
Did you press the dose-release button when turning the clear base? Close the cap, so the dose-release button is covered, then turn the clear base.
Did you stop when turning the clear base before it clicked? Turn the clear base in a continuous movement until it clicks (half a turn).
My COMBIVENT RESPIMAT doesn’t spray:
Did you insert a cartridge? If not, insert a cartridge.
Did you repeat Turn, Open, Press (TOP) less than three times after inserting the cartridge? Repeat Turn, Open, Press (TOP) three times after inserting the cartridge as shown in steps 4 to 6 under “Prepare for first use”.
Is the dose indicator on the COMBIVENT RESPIMAT pointing to 0 (zero)? You have used up all your medicine and the inhaler is locked.
For more information about COMBIVENT RESPIMAT including current prescribing information or a video demonstration on how to use COMBIVENT RESPIMAT, go to www.combivent.com, scan the code, or call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257.

This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT 06877 USA
Licensed from: Boehringer Ingelheim International GmbH
RESPIMAT® is a registered trademark of and used under license from Boehringer Ingelheim International GmbH
COMBIVENT® is a registered trademark of and used under license from Boehringer Ingelheim Pharmaceuticals, Inc.
Copyright © 2021 Boehringer Ingelheim International GmbH
- PRINCIPAL DISPLAY PANEL - 20 mcg/100 mcg Carton
-
INGREDIENTS AND APPEARANCE
COMBIVENT RESPIMAT
ipratropium bromide and albuterol spray, meteredProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0597-0024 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IPRATROPIUM BROMIDE (UNII: J697UZ2A9J) (IPRATROPIUM - UNII:GR88G0I6UL) IPRATROPIUM BROMIDE ANHYDROUS 20 ug ALBUTEROL SULFATE (UNII: 021SEF3731) (ALBUTEROL - UNII:QF8SVZ843E) ALBUTEROL 100 ug Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0597-0024-02 1 in 1 CARTON 07/01/2012 1 120 in 1 CARTRIDGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021747 07/01/2012 Labeler - Boehringer Ingelheim Pharmaceuticals Inc. (603175944) Registrant - Boehringer Ingelheim Pharmaceuticals Inc. (603175944) Establishment Name Address ID/FEI Business Operations Boehringer Ingelheim Pharma GmbH and Co. KG 551147440 API MANUFACTURE(0597-0024) , LABEL(0597-0024) , MANUFACTURE(0597-0024) , PACK(0597-0024)