Label: PROIN 75- phenylpropanolamine hydrochloride tablet, chewable
- NDC Code(s): 49427-316-48, 49427-316-50
- Packager: Pegasus Laboratories, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated January 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
Description:
PROIN (phenlypropanolamine hydrochloride) is a sympathomimetic amine closely related to ephedrine.
Phenylpropanolamine hydrochloride (PPA) is the nonproprietary designtion for benzenemethanol, α - (1-aminoethyl) - hydrochloride, (R*,S*)-, (±).
The empirical formula is C9H13NO•HCL and the molecular weight is 187.67. It is a white cyrstalline compound having a slight aromatic odor.
PPA is freely soluable in water and alcohol but is practically insoluable in ether, benzene and chloroform. The chemical structure of phenylpropanolamine is:
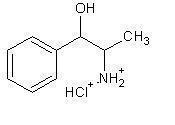
- Indication:
- Dosage and Administration:
- Warnings:
-
Precautions:
PROIN may cause increased thirst; therefore, provide ample fresh water.
Overdose has been associated with dogs chewing through closed bottles of PROIN and consuming multiple tablets. Therefore, it is important to store PROIN Chewable Tablets out of reach of dogs and other pets in a secured location.
Use in dogs with incontinence due to a urinary tract infection will mask symptoms. PROIN is not effective in dogs with incontinence due to neurologic disease or malformations.
PROIN may cause hypertension; therefore, use with caution in dogs with pre-existing heart disease, hypertension, liver disease, kidney insufficiency, diabetes, glaucoma, and conditions with a predilection for hypertension. Use with caution in dogs receiving sympathomimetic drugs, tricyclic antidepressants, or monoamine oxidase inhibitors as increased toxicity may result. Use with caution in dogs administered halogenated gaseous anesthetics as this may increase the risk of cardiac arrhythmias.
A laboratory study on human blood revealed that PPA used in conjunction with aspirin may potentiate decreased platelet aggregation.
The safe use of PROIN in dogs used for breeding purposes, during pregnancy or in lactating bitches has not been evaluated.
-
Adverse Reactions "Pre Approval Experience":
A placebo controlled clinical study involving 123 PROIN treated dogs and 61 placebo treated dogs was conducted for 28 days. The most common adverse reactions are shown in Table 1 below. In addition, one dog exhibited disorientation, nervousness, a 7.7% loss of body weight, and hypertension with proteinuria. A second dog exhibited restless behavior, lethargy, a 2.8% body weight loss and proteinuria.
Table 1: Number and percentage of dogs with adverse reactions in the 28 day placebo-controlled clinical study
Adverse Reactions PROIN Treated (N=123) Placebo Treated (N=61) Emesis 20.3% 8.2% Hypertension (≥ 160mm Hg)1 19.5% 14.7% Anorexia 16.3% 3.3% Body weight loss (> 5%) 2 16.1% 6.8% Proteinuria 13.0% 8.2% Anxiety/aggression/behavior change 9.7% 3.2% Diarrhea 7.3% 9.8% Polydipsia 6.5% 9.8% Lethargy 5.7% 1.6% Musculoskeletal Disorder 3.2% 1.6% Insomnia/Sleep Disorder 2.5% 0.0% 1 One or more systolic blood pressure readings of ≥ 160mmHg.
2 The "N" for weight loss is PROIN treated N=118 and placebo N=59 because seven dogs did not have a final weight at the time of withdrawal from the study.
-
ADVERSE REACTIONS
One-hundred fifty seven dogs continued into the 6 month open label clinical study. The most common adverse reactions are listed in Table 2 below. In addition, one dog exhibited progressively worsening hypertension with proteinuria. Five dogs enrolled in the study with pre-existing heart disease. Of these, one dog developed systolic failure with an unknown relation to treatment.
-
ADVERSE REACTIONS
Table 2: Number and percentage of dogs with adverse reactions in the 6-month open-label clinical study
Adverse Reactions
Total N= 125 Hypertension (≥ 160 mmHg)1
34.6%
Body Weight Loss (> 5%)
24.8%
Emesis
19.7%
Proteinuria
15.3%
Anorexia
10.2%
Diarrhea
6.4%
Lethargy
5.7%
Anxiety/ behavior change/ aggression
5.7%
1 Percent of dogs with systolic blood pressures of ≥ 160 mmHg on day 7 were 30.2% and on day 0 were 33.3%.
-
POST APPROVAL EXPERIENCE (2015):
The following adverse events are based on voluntary, post approval reporting. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data. The signs reported are listed in decreasing order of reporting frequency by body system:
Gastrointestinal: Vomiting, anorexia, diarrhea, hypersalivation,
Behavioral: Agitation, lethargy, vocalization, confusion,
General body system: Polydipsia, weight loss, weakness, fever,
Respiratory: Panting,
Dermatological: Erythema, piloerection,
Hepatic: Elevated serum alanine aminotransferase (ALT), elevated serum alkaline phosphatase (ALP),
Neurologic: Ataxia, seizures, tremors,
Renal/Urinary: Renal failure, hematuria, urinary retention,
Cardiovascular: Tachycardia, hypertension, bradycardia, arrhythmias,
Sensory: Ophthalmic disorders, mydriasis and eye redness.
In some cases, death, including euthanasia, has been reported. Sudden death was sometimes preceded by neurologic signs, vocalization, or collapse. A necropsy of one dog revealed subarachnoidal and intraventricular hemorrhage in the brain.
The following signs have been reported more often with a dose higher than the recommended dosage: agitation, arrhythmia, bradycardia, erythema, fever, hypersalivation, hypertension, lethargy, mydriasis, panting, piloerection, tachycardia, tremor, and urinary retention.
For a copy of the Safety Data Sheet (SDS) or to report suspected adverse drug events, contact Pegasus Laboratories at 1-800-874-9764. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
-
Information for Owner or Person Treating Animal:
Always follow the dosage instructions for PROIN provided by your veterinarian. Monitor your dog after giving PROIN to be sure all of it was consumed. If you have difficulty giving PROIN, contact your veterinarian.
It may take several days of treatment with PROIN before urinary incontinence improves. If you miss a dose, give it as soon as you remember. If it is close to the time for the next dose, skip the dose you missed and go back to the regular dosing schedule. Do not give two doses at once. PROIN should only be given to the dog for which it was prescribed. Because PROIN is flavored, store in a secure area.
Dogs may willingly consume more than the recommended dosage of PROIN Chewable Tablets. Instances of dogs chewing through closed bottles of PROIN and eating the bottles contents have been reported. Keep the product in a secured storage area out of the reach of pets in order to prevent accidental ingestion or overdose. Contact your veterinarian immediately if the dog ingests more tablets than prescribed or if other pets ingest PROIN Chewable Tablets. In the case of accidental ingestion by humans, contact a physician immediately.
Contact your veterinarian if you notice restlessness or irritability, loss of appetite, the incontinence persists or worsens, or any other unusual signs.
Consult your veterinarian before using PROIN with any other medications.
-
Clinical Pharmacology:
Phenylpropanolamine is a chemical analogue of the endogenous sympathomimetic amines. It is an alpha-adrenergic agent which has been reported to increase urethral tone in dogs. 2 Its mechanism of action is not well determined, but it is believed to cause the release of norepinephrine by indirectly stimulating both the alpha and beta-adrenergic receptors of the smooth muscle to increase smooth muscle tone of the urethra, bladder neck, and the internal urethral sphincter. 3, 4
The pharmacokinetics of phenylpropanolamine in dogs has not been well studied. In humans, phenylpropanolamine is readily absorbed after oral administration of solid dosage forms and has an onset of action of approximately 15-30 minutes and duration of effect of about three hours. In a published study in dogs, phenylpropanolamine disposition was characterized in three dogs administered phenylpropanolamine intravenously and orally in immediate-release and controlled-release formulations. 5 The terminal elimination half-life averaged 3.5 ± 0.5 hours after the intravenous dose. Oral absorption from the immediate-release capsule was rapid and bioavailability was 98.2 ± 6.9 percent. Absorption of phenylpropanolamine from the controlled-release dosage form was biphasic; an initial rapid phase was followed by a second, slower absorption phase which continued over 16 hours. Plasma concentrations then declined with a half-life roughly parallel to the intravenous and oral immediate-release half-lives. Oral bioavailability from the controlled-release tablet was 93.7 ± 5.9 percent.
-
Effectiveness:
A 28-day placebo-controlled clinical study was conducted in 21 study sites across the U.S. The study included 184 dogs with urinary incontinence due to sphincter hypotonus of which 127 dogs (100 female, 27 male) were evaluated for effectiveness. Dogs were randomly assigned either to receive 2 mg/kg PROIN (123 dogs) or placebo (61 dogs) administered twice daily for 28 days. PROIN was effective in controlling urinary incontinence based on decrease in urinary accidents per week. Changes to hematology and serum chemistry were not considered clinically significant or related to treatment.
Table 3: Mean urinary accidents per week by treatment group, females
Week Mean Urinary Accidents (PROIN-treated, N=66) Mean Urinary Accidents (Placebo-treated, N=34) Pretreatment 9.0 7.8 1 3.9 4.8 2 2.5 4.1 3 1.5 3.1 4 1.6 2.8 One-hundred fifty seven dogs continued into the 6-month open label clinical study conducted in 21 study sites across the U.S. All the dogs had participated in the 28-day placebo-controlled clinical study and had urinary incontinence due to sphincter hypotonus. Dogs were administered 2 mg/kg PROIN orally twice daily for 180 days. PROIN was effective for the control of urinary incontinence for 180 days based on 98.1% owner satisfaction. The dogs averaged just one accident per dog per week. Changes to hematology and serum chemistry were not considered clinically significant or related to treatment.
The dogs voluntarily consumed 53.9% of the doses and 33.7% of the doses in food. The owners pilled the dogs 12.1% of the doses and were unable to administer 0.3% of the doses.
-
Animal Safety Studies:
In a target animal safety study, PROIN was administered to 32 healthy male and female Beagle dogs at 0, 2, 6 and 10 mg/kg of body weight (0, 1, 3, and 5 times the recommended dose; 8 dogs per group) twice daily for 26 consecutive weeks. The most pronounced finding was a dose-dependent increase in blood pressure. Mean systolic blood pressure was increased in all PPA-treated groups compared to the control, but mean values for all 4 groups were within the normal range. Mean diastolic and mean MAP (mean arterial pressure) were higher in the 3X and 5X groups, and in the 1X males. Dogs in the 3X and 5X groups had more individual systolic, diastolic, and MAP values above the normal range than the control group dogs. A dose-dependent decrease in heart rate was observed in the 3X and 5X dogs. In the 0, 1, 3, and 5X groups, 5%, 34%, 44% and 40% of the total number of heart rates obtained from electrocardiograms for each group over the course of the study were below the normal range (70-120 beats per minute), with the lowest value being 51 bpm in 4 of the 1X group dogs. One dog in each of the 1X and 5X had an elevated heart rate between 150-180 beats per minute on at least 2 of the 13 physical exams. One dog in each of the 1X and 3X groups developed gallop heart sounds after treatment began that were noted in 12 of 13 and 6 of 13 physical exams respectively. Dogs in the PPA-treated group exhibited anxious/ restless behavior more frequently than the control group. One dog each in the 1X and 3X were responsible for the majority of the observations. A decline in mean body weight and body condition was observed in females in all 4 groups, including the control. One female in the 1X group lost 33% body weight. Vomiting and loose stool occurred in a dose-related fashion, and most of the vomiting episodes took place within 1 hour of dosing. Mean platelet counts were higher in at least one of the PPA treated groups, with individual values up to 1.4X the upper limit of normal (ULN) in the 3X and 5X group. The 3X and 5X groups had higher mean serum ALT values compared to the control. Mean ALT was within the normal range for all 4 groups. There were more dogs with ALT levels above the normal range in the 3X PPA treated compared to the control, but increased values were transient and less than 1.8X ULN. All dogs had ALT values in the normal range at the conclusion of the study.
In a separate tolerance study, 6 healthy female Beagle dogs were administered PROIN at 20 mg/kg body weight (10 times the recommended dose) twice daily for 21 consecutive days. Mean systolic blood pressure was increased in the 10X group compared to the control, but mean values were within the normal range for both groups. Mean diastolic pressures were above the normal range on days 7 and 21 for the 10X group, and day 14 for the control. The 10X dogs had hypertensive MAP values on days 7 and 21, whereas the control dog mean MAP values were in the normal range. There was a trend in 10X dogs for lower heart rates following initiation of PPA treatment. Four of 6 dogs in the 10X group had heart rates below the normal range on day 7, whereas none of the control dogs did. The 10X group dogs had increased hematocrit, hemoglobin, RBC counts, urine specific gravity, and water intake consistent with transient, sub-clinical dehydration that occurred shortly after PPA treatment was started. All 6 dogs in the 10X group vomited at least once during the treatment period, whereas only 1 of the control dogs did. Most of the vomiting episodes took place within 1 hour of dosing. Mean platelet counts were also higher in the 10X dogs on all 3 exam days; mean values were above the normal range on day 7, with individual values up to 1.5X ULN. The 10X group had a higher mean serum ALT value on day 7 than the control. Mean ALT values for both groups were in the normal range on all 3 exam days, but 2 dogs in the 10X group had ALT values up to 1.4X ULN on day 7; these elevated values were transient, and all dogs had normal ALT values on days 14 and 21.
For either study, there was no evidence of chronic hypertension-induced target organ damage; there were no clinical findings attributed to PPA on the ophthalmic exams, electrocardiogram evaluation, or gross necropsy and histopathology.
- Storage:
- How Supplied:
-
References:
1 Watson R, et. al. Ephedra alkaloids inhibit platelet aggregation. Blood Coagulation and Fibrinolysis. 2010 21: 266-271
2 Richter K. P., Ling G.V. Clinical response and urethral pressure profile changes after phenylpropanolamine in dogs with primary sphincter incompetence. JAVMA Vol 187 No. 6, September 15, 1985, 605-611.
3 Scott, L., Leddy M., and Bernay F. Evaluation of phenylpropanolamine in the treatment of urethral sphincter mechanism incompetence in the bitch. J. Small Animal Pract. 2002; 43 (11): 493-6.
4 Noel, S., et. al. Combined pharmacokinetic and urodynamic study of the effects of oral administration of phenylpropanolamine in female Beagle dogs. Vet Journal, 2010; 184 (2): 201-207.
5 Hussain, M.A., Aungst, B.J., Lam, G., and Shefter, E. Phenylpropanolamine pharmacokinetics in dogs after intravenous, oral, and oral controlled-release doses. Biopharm Drug Dispos, Vol. 8, No. 5, September-October 1987, 497-505.
-
BOXED WARNING
(What is this?)
Warnings: Keep out of reach of children. Not for human use. Consult a physician in case of accidental ingestion by humans. For use in dogs only.
Precautions: Store PROIN Chewable tablets out of reach of dogs and other pets in a secured location. Instances of dogs chewing through closed bottles of PROIN and eating the bottles contents have been reported. If accidental exposure occurs, consult a veterinarian and notify Pegasus Laboratories (1-800-874-9764). See Product Insert for complete information.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PROIN 75
phenylpropanolamine hydrochloride tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:49427-316 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLPROPANOLAMINE HYDROCHLORIDE (UNII: 8D5I63UE1Q) (PHENYLPROPANOLAMINE - UNII:33RU150WUN) PHENYLPROPANOLAMINE HYDROCHLORIDE 75 mg Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) SOY PROTEIN (UNII: R44IWB3RN5) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) WHEY (UNII: 8617Z5FMF6) GARLIC (UNII: V1V998DC17) Product Characteristics Color brown Score 2 pieces Shape ROUND (Scored on one side imprint code on other side) Size 16mm Flavor LIVER Imprint Code Proin;75 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49427-316-48 60 in 1 BOTTLE 2 NDC:49427-316-50 180 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141324 10/10/2011 Labeler - Pegasus Laboratories, Inc. (108454760) Registrant - Pegasus Laboratories, Inc. (108454760) Establishment Name Address ID/FEI Business Operations Pegasus Laboratories, Inc. 108454760 manufacture, analysis, label




