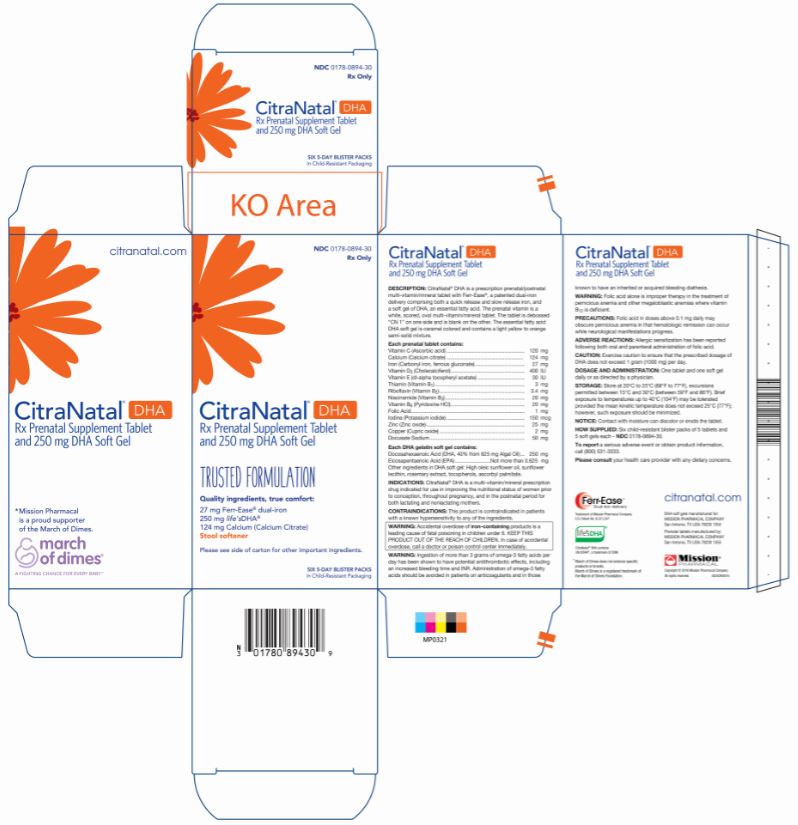
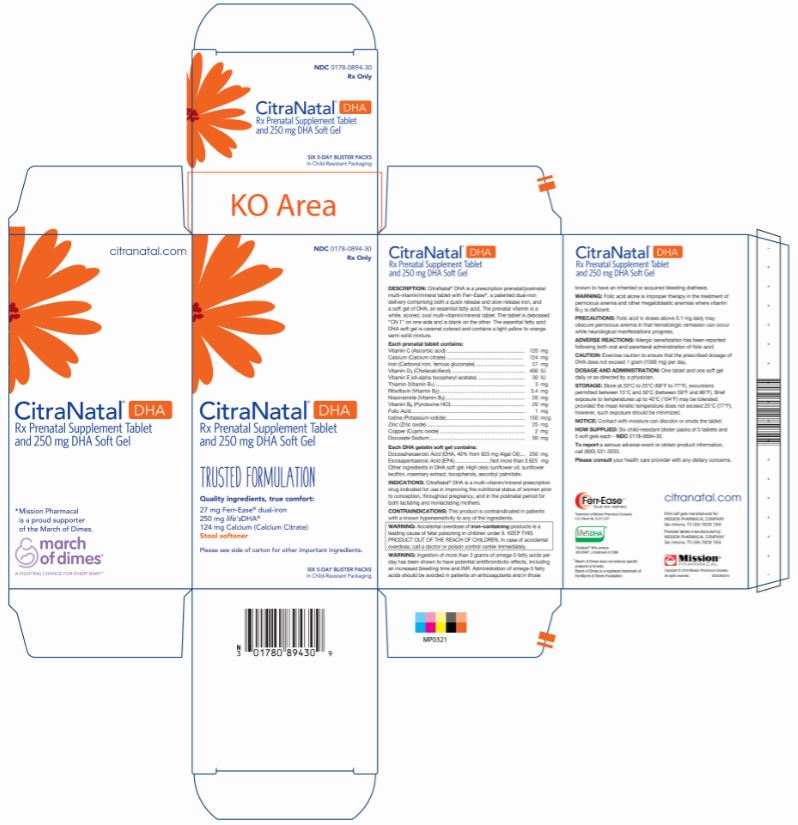
Label: CITRANATAL DHA- vitamin c, calcium, iron, vitamin d3, vitamin e, thiamin, riboflavin, niacinamide, vitamin b6, folic acid, iodine, zinc, copper, docusate sodium kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 0178-0894-30 - Packager: Mission Pharmacal Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated July 28, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- BOXED WARNING (What is this?)
- KEEP OUT OF REACH OF CHILDREN
-
DESCRIPTION
CitraNatal ® DHA is a prescription prenatal/postnatal multi-vitamin/mineral tablet with Ferr-Ease ®, a patented dual-iron delivery comprising both a quick release and slow release iron, and a soft gel of an essential fatty acid. The prenatal vitamin is a white, scored, oval multi-vitamin/mineral tablet. The tablet is debossed “CN 1” on one side and is blank on the other. The essential fatty acid DHA soft gel is caramel colored and contains a light yellow to orange semi-solid mixture.
-
ACTIVE INGREDIENT
Each prenatal tablet contains: Vitamin C (Ascorbic acid) 120 mg Calcium (Calcium citrate) 124 mg Iron (Carbonyl iron, ferrous gluconate) 27 mg Vitamin D3 (Cholecalciferol) 400 IU Vitamin E (dl-alpha tocopheryl acetate) 30 IU Thiamin (Vitamin B1) 3 mg Riboflavin (Vitamin B2) 3.4 mg Niacinamide (Vitamin B3) 20 mg Vitamin B6 (Pyridoxine) 20 mg Folic Acid 1 mg Iodine (Potassium iodide) 150 mcg Zinc (Zinc oxide) 25 mg Copper (Cupric oxide) 2 mg Docusate Sodium 50 mg Each DHA soft gel contains: Other ingredients in DHA soft gel: High oleic sunflower oil, sunflower lecithin, rosemary extract, tocopherols, ascorbyl palmitate. Docosahexaenoic Acid (DHA, 40% from 625 mg Algal Oil) 250 mg Eicosapentaenoic Acid (EPA) Not more than 0.625 mg - PURPOSE
- INACTIVE INGREDIENT
- INDICATIONS
-
WARNINGS
WARNING:
Ingestion of more than 3 grams of omega-3 fatty acids per day has been shown to have potential antithrombotic effects, including an increased bleeding time and INR. Administration of omega-3 fatty acids should be avoided in patients on anticoagulants and in those known to have an inherited or acquired bleeding diathesis.WARNING:
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B 12 is deficient. - PRECAUTIONS
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
-
STORAGE
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (between 59°F and 86°F). Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimalized.
NOTICE: Contact with moisture can discolor or erode the tablet.
-
HOW SUPPLIED
Six child-resistant blister packs of 5 tablets and 5 soft gels each - NDC 0178-0894-30.
To report a serious adverse event or obtain product information, call (210) 696-8400.
Please consult your health care provider with any dietary concerns.
DHA soft gels manufactured for:
MISSION PHARMACAL COMPANY
San Antonio, TX USA 78230 1355
Prenatal tablets manufactured by:
MISSION PHARMACAL COMPANY
San Antonio, TX USA 78230 1355Copyright © 2014 Mission Pharmacal Company.
All rights reserved. - PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CITRANATAL DHA
vitamin c, calcium, iron, vitamin d3, vitamin e, thiamin, riboflavin, niacinamide, vitamin b6, folic acid, iodine, zinc, copper, docusate sodium kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0178-0894 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0178-0894-30 30 in 1 CARTON 05/22/2014 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 Part 2 1 Part 1 of 2 PRENATAL VITAMIN
vitamin c, calcium, iron, vitamin d3, vitamin e, thiamin, riboflavin, niacinamide, vitamin b6, folic acid, iodine, zinc, copper, docusate sodium tabletProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 120 mg VITAMIN D (UNII: 9VU1KI44GP) (CHOLECALCIFEROL - UNII:1C6V77QF41) VITAMIN D 400 [iU] IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 27 mg .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) (.ALPHA.-TOCOPHEROL - UNII:H4N855PNZ1) .ALPHA.-TOCOPHEROL 30 [iU] THIAMINE (UNII: X66NSO3N35) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 3 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3.4 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 20 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg POTASSIUM IODIDE (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) POTASSIUM IODIDE 150 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 25 mg CUPRIC OXIDE (UNII: V1XJQ704R4) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 2 mg DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg CALCIUM CITRATE (UNII: MLM29U2X85) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CITRATE 124 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MAGNESIUM SILICATE (UNII: 9B9691B2N9) SHELLAC (UNII: 46N107B71O) ETHYL VANILLIN (UNII: YC9ST449YJ) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) Product Characteristics Color white (white to off white) Score no score Shape OVAL (modified oval) Size 20mm Flavor Imprint Code CN;1 Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/22/2014 Part 2 of 2 DHA
doconexent and icosapent capsuleProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCONEXENT (UNII: ZAD9OKH9JC) (DOCONEXENT - UNII:ZAD9OKH9JC) DOCONEXENT 250 mg ICOSAPENT (UNII: AAN7QOV9EA) (ICOSAPENT - UNII:AAN7QOV9EA) ICOSAPENT 0.625 mg Inactive Ingredients Ingredient Name Strength SUNFLOWER OIL (UNII: 3W1JG795YI) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) ROSEMARY (UNII: IJ67X351P9) TOCOPHEROL (UNII: R0ZB2556P8) ASCORBYL PALMITATE (UNII: QN83US2B0N) Product Characteristics Color brown (caramel) Score no score Shape CAPSULE Size 23mm Flavor Imprint Code Contains Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 05/22/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/22/2014 Labeler - Mission Pharmacal Company (008117095) Registrant - Mission Pharmacal Company (927726893) Establishment Name Address ID/FEI Business Operations Mission Pharmacal Company 927726893 manufacture(0178-0894)