Label: PROMACE- acepromazine maleate injection
- NDC Code(s): 0010-3827-01
- Packager: Boehringer Ingelheim Animal Health USA Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated January 5, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Caution:
-
Description:
PROMACE Injectable, a potent neuroleptic agent with a low order of toxicity, is of particular value in the tranquilization of dogs, cats and horses. Its rapid action and lack of hypnotic effect are added advantages. According to Baker,1 the scope of possible applications for this compound in veterinary practice is only limited by the imagination of the practitioner.
Each mL contains: acepromazine maleate 10 mg, sodium citrate 0.36%, citric acid 0.075%, benzyl alcohol 1% and water for injection.
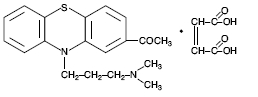
Acepromazine [10-[3-(dimethylamino) propyl] phenothiazin-2-yl-methyl ketone] Maleate, USP has the following chemical structure:
PROMACE Injectable has a depressant effect on the central nervous system and, therefore, causes sedation, muscular relaxation and a reduction in spontaneous activity. It acts rapidly, exerting a prompt and pronounced calming effect.
-
Indications:
Dogs and Cats: PROMACE Injectable can be used as an aid in controlling intractable animals during examination, treatment, grooming, x-ray and minor surgical procedures; to alleviate itching as a result of skin irritation; as an antiemetic to control vomiting associated with motion sickness.
PROMACE Injectable is particularly useful as a preanesthetic agent (1) to enhance and prolong the effects of barbiturates, thus reducing the requirements for general anesthesia; (2) as an adjunct to surgery under local anesthesia.
Horses: PROMACE Injectable can be used as an aid in controlling fractious animals during examination, treatment, loading and transportation. Particularly useful when used in conjunction with local anesthesia for firing, castration, neurectomy, removal of skin tumors, ocular surgery and applying casts.
-
Dosage and Administration:
The dosage should be individualized, depending upon the degree of tranquilization required. As a general rule, the dosage requirement in mg/lb of body weight decreases as the weight of the animal increases.
PROMACE Injectable may be given intravenously, intramuscularly or subcutaneously. The following schedule may be used as a guide to IV, IM or SC injections:
-
Dogs: 0.25–0.5 mg/lb of body weight
Cats: 0.5–1 mg/lb of body weight
Horses: 2–4 mg/100 lb of body weight
IV doses should be administered slowly, and a period of at least 15 minutes should be allowed for the drug to take full effect.
-
Contraindications:
Phenothiazines may potentiate the toxicity of organophosphates and the activity of procaine hydrochloride. Therefore, do not use PROMACE Injectable to control tremors associated with organic phosphate poisoning. Do not use in conjunction with organophosphorus vermifuges or ectoparasiticides, including flea collars. Do not use with procaine hydrochloride.
- Warning:
-
Precautions:
Tranquilizers are potent central nervous system depressants and they can cause marked sedation with suppression of the sympathetic nervous system.
Tranquilizers can produce prolonged depression or motor restlessness when given in excessive amounts or when given to sensitive animals.
Tranquilizers are additive in action to the actions of other depressants and will potentiate general anesthesia. Tranquilizers should be administered in smaller doses and with greater care during general anesthesia and also to animals exhibiting symptoms of stress, debilitation, cardiac disease, sympathetic blockade, hypovolemia or shock. PROMACE Injectable, like other phenothiazine derivatives, is detoxified in the liver; therefore, it should be used with caution in animals with a previous history of liver dysfunction or leukopenia.
Hypotension can occur after rapid intravenous injection causing cardiovascular collapse.
Epinephrine is contraindicated for treatment of acute hypotension produced by phenothiazine-derivative tranquilizers since further depression of blood pressure can occur. Other pressor amines, such as norepinephrine or phenylephrine, are the drugs of choice.
In horses, paralysis of the retractor penis muscle has been associated with the use of phenothiazine-derivative tranquilizers. Such cases have occurred following the use of PROMACE Injectable. This risk should be duly considered prior to the administration of PROMACE Injectable to male horses (castrated and uncastrated). When given, the dosage should be carefully limited to the minimum necessary for the desired effect. At the time of tranquilization, it is not possible to differentiate between reversible protrusion of the penis (a normal clinical sign of narcosis) and the irreversible paralysis of the retractor muscle. The cause of this side reaction has not been determined. It has been postulated that such paralysis may occur when a tranquilizer is used in conjunction with testosterone (or in stallions).
Accidental intracarotid injection in horses can produce clinical signs ranging from disorientation to convulsive seizures and death.
-
Adverse Reactions:
A few rare but serious occurrences of idiosyncratic reactions to acepromazine may occur in dogs following oral or parenteral administration. These potentially serious adverse reactions include behavioral disorders in dogs such as aggression, biting/chewing, and nervousness.
To report suspected adverse reactions, to obtain a Safety Data Sheet or for technical assistance, call 1-888-637-4251.
For additional information about adverse drug experience reporting for animal drugs, contact FDA by telephone at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
-
Animal Safety:
Acute and chronic toxicity studies have shown a very low order of toxicity.
Acute toxicity: The LD50 dose of PROMACE Injectable in mice was determined by means of a probit transformation with the following results:2
- Intravenous route — 61.37 mg/kg
Subcutaneous route — 130.5 mg/kg
Oral route — 256.8 mg/kg
Chronic toxicity: Tests3 in rats revealed no deleterious effects on renal or hepatic function or on hemopoietic activity. In several groups of two male and two female beagle hounds treated for six months with daily oral doses of 20 to 40 mg/kg, no untoward effects were encountered. Hematologic studies and urinalysis gave values within normal limits. Another group of four dogs, given gradually increasing oral doses up to a level of 220 mg/kg daily and reaching a total daily dose of 2.2 g per dog, showed some signs of pulmonary edema and hyperemia of the internal organs, but no animal died.
When administered intramuscularly, PROMACE Injectable causes a brief sensation of stinging comparable with that observed with other phenothiazine tranquilizers.
-
Effectiveness:
Controlled clinical studies in the United States and Canada have demonstrated the effectiveness and safety of PROMACE Injectable as a tranquilizer.
Good to excellent results were reported1,4,5 in dogs, cats and horses given PROMACE Injectable for restraint during examination, treatment and minor surgery and for preanesthetic sedation. In dogs, the drug reportedly4 helps control convulsions associated with distemper.
In both dogs and cats, good to excellent results were obtained4 when PROMACE Tablets were used to control nervousness, excessive vocalization, neurotic and excitable behavior, vomiting associated with motion sickness, coughing and itching caused by dermatitis.
In horses, Bauman6 had good results using the drug as an aid in the control of painful spasms due to colic.
Other practitioners7,8 found the drug useful as a preanesthetic sedative for nervous or aggressive horses, but it had to be administered while the animals were quiet and not in an excited state. In a trial9 on more than 200 horses with a wide variety of disorders, PROMACE Injectable proved to be both effective and safe.
- Storage:
- How Supplied:
-
References:
1. Baker, J.M.: Paper presented at the Ontario Veterinary Association meeting, held in Toronto, Canada,1958.
2. Pharmacology Reports, ClinByla Laboratories, Paris, France.
3. Stegen, M.G.: Pharmacology Report, Ayerst Laboratories, 1958.
4. Veterinary Medical Records, Ayerst Laboratories.
5. Foley, J.T.: Clinical Reports to Ayerst Laboratories, 1963.
6. Bauman, W.G.: Clinical Reports to Ayerst Laboratories, 1963.
7. Ford, R.W.: in Equine Panel Report, Mod. Vet. Pract. 40:45 (Nov. 1) 1959.
8. Baldwin, R.: in Equine Panel Report, Mod. Vet. Pract. 40:46 (Nov. 1) 1959.
9. Dunkin, T.E.: Clinical Reports to Ayerst Laboratories, 1963.
- SPL UNCLASSIFIED SECTION
- Principal Display Panel – 50 mL Container Label
-
INGREDIENTS AND APPEARANCE
PROMACE
acepromazine maleate injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0010-3827 Route of Administration INTRAVENOUS, INTRAMUSCULAR, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACEPROMAZINE MALEATE (UNII: 37862HP2OM) (ACEPROMAZINE - UNII:54EJ303F0R) ACEPROMAZINE MALEATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) 10 mg in 1 mL TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) 3.6 mg in 1 mL ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) 0.75 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0010-3827-01 50 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA015030 06/07/2011 Labeler - Boehringer Ingelheim Animal Health USA Inc. (007134091)