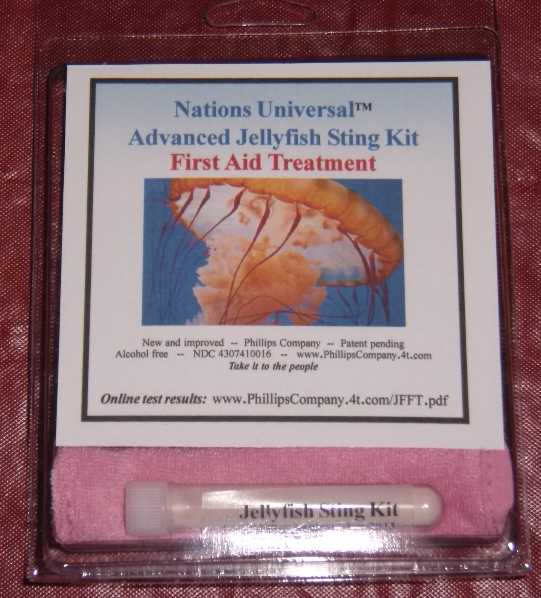
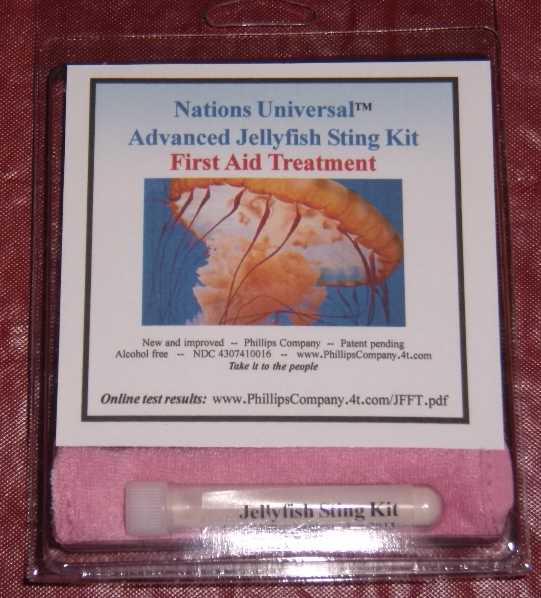
Label: ADVANCED JELLYFISH STING KIT- zinc acetate powder, for suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 43074-114-16, 43074-114-17, 43074-114-18 - Packager: Phillips Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 12, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DRUG FACTS
Uses
Temporarily protects injured or exposed skin or mucous membrane surfaces from harmful or annoying stimuli, and may help provide relief to such surfaces. Contains surfactants to break down oily toxins.
____________________________________________________________________
Warnings
n For external use only; do not swallow. n Keep away from children.
n Do not use in the eyes or apply over large areas of the body.
n Do not use if allergic to any ingredient listed on this label.
____________________________________________________________________
Directions
Step 1: Wet the skin with water and apply powder to the affected area. Step 2: Use water to wet the special cloth and use it to scrub the affected area to remove jellyfish barbs and to mix the protectant with any remaining toxins. Step 3: Repeat Steps 1 and 2 a minimum of three times or until stinging and pain stops. Step 4: Seek immediate medical attention.
____________________________________________________________________
Other information
Store at 40 to 120 degrees F. Net contents of kit: One special scrub cloth and one powder vial. Net contents of powder vial: 3 mL. This product contains no alcohol; no animal products; no biological products.
____________________________________________________________________
Inactive ingredients
Water, calcium carbonate, sodium hypochlorite, sodium carbonate, sodium dodecylbenzene sulfonate, ascorbic acid, magnesium stearate, sorbic acid, stearic acid, diproplyene glycol, dimethyl sulfoxide, color.
____________________________________________________________________
Questions and Side effects
Report any side effects to Phillips Company, 311 Chickasaw St, Millerton, OK USA 74750
Email: phillips@123phillips.com
- ACTIVE INGREDIENTS
-
ASK DOCTOR
Directions
Step 1: Wet the skin with water and apply powder to the affected area. Step 2: Use water to wet the special cloth and use it to scrub the affected area to remove jellyfish barbs and to mix the protectant with any remaining toxins. Step 3: Repeat Steps 1 and 2 a minimum of three times or until stinging and pain stops. Step 4: Seek immediate medical attention. - DO NOT USE
- CHILDREN
- PURPOSE
- STOP USE
-
WHEN USING
Directions
Step 1: Wet the skin with water and apply powder to the affected area. Step 2: Use water to wet the special cloth and use it to scrub the affected area to remove jellyfish barbs and to mix the protectant with any remaining toxins. Step 3: Repeat Steps 1 and 2 a minimum of three times or until stinging and pain stops. Step 4: Seek immediate medical attention.
Store at 40 to 120 degrees F.
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
Step 1: Wet the skin with water and apply powder to the affected area. Step 2: Use water to wet the special cloth and use it to scrub the affected area to remove jellyfish barbs and to mix the protectant with any remaining toxins. Step 3: Repeat Steps 1 and 2 a minimum of three times or until stinging and pain stops. Step 4: Seek immediate medical attention.
- USE
- INACTIVE INGREDIENTS
- Image of product
-
INGREDIENTS AND APPEARANCE
ADVANCED JELLYFISH STING KIT
zinc acetate powder, for suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43074-114 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength zinc acetate (UNII: FM5526K07A) (zinc - UNII:J41CSQ7QDS) zinc 0.001 mg in 1 mL Inactive Ingredients Ingredient Name Strength dimethyl sulfoxide (UNII: YOW8V9698H) ascorbic acid (UNII: PQ6CK8PD0R) dipropylene glycol (UNII: E107L85C40) water (UNII: 059QF0KO0R) sorbic acid (UNII: X045WJ989B) magnesium stearate (UNII: 70097M6I30) stearic acid (UNII: 4ELV7Z65AP) sodium dodecylbenzenesulfonate (UNII: 554127163Y) calcium carbonate (UNII: H0G9379FGK) sodium carbonate (UNII: 45P3261C7T) sodium hypochlorite (UNII: DY38VHM5OD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43074-114-18 1 in 1 PACKAGE, COMBINATION 1 NDC:43074-114-16 3 mL in 1 VIAL, PLASTIC 2 NDC:43074-114-17 0.003 mL in 1 APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 11/12/2010 Labeler - Phillips Company (612368238) Establishment Name Address ID/FEI Business Operations Phillips Company 612368238 manufacture