Label: 3M AVAGARD D- alcohol lotion
- NDC Code(s): 17518-050-00, 17518-050-01, 17518-050-02
- Packager: Solventum US OpCo LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
-
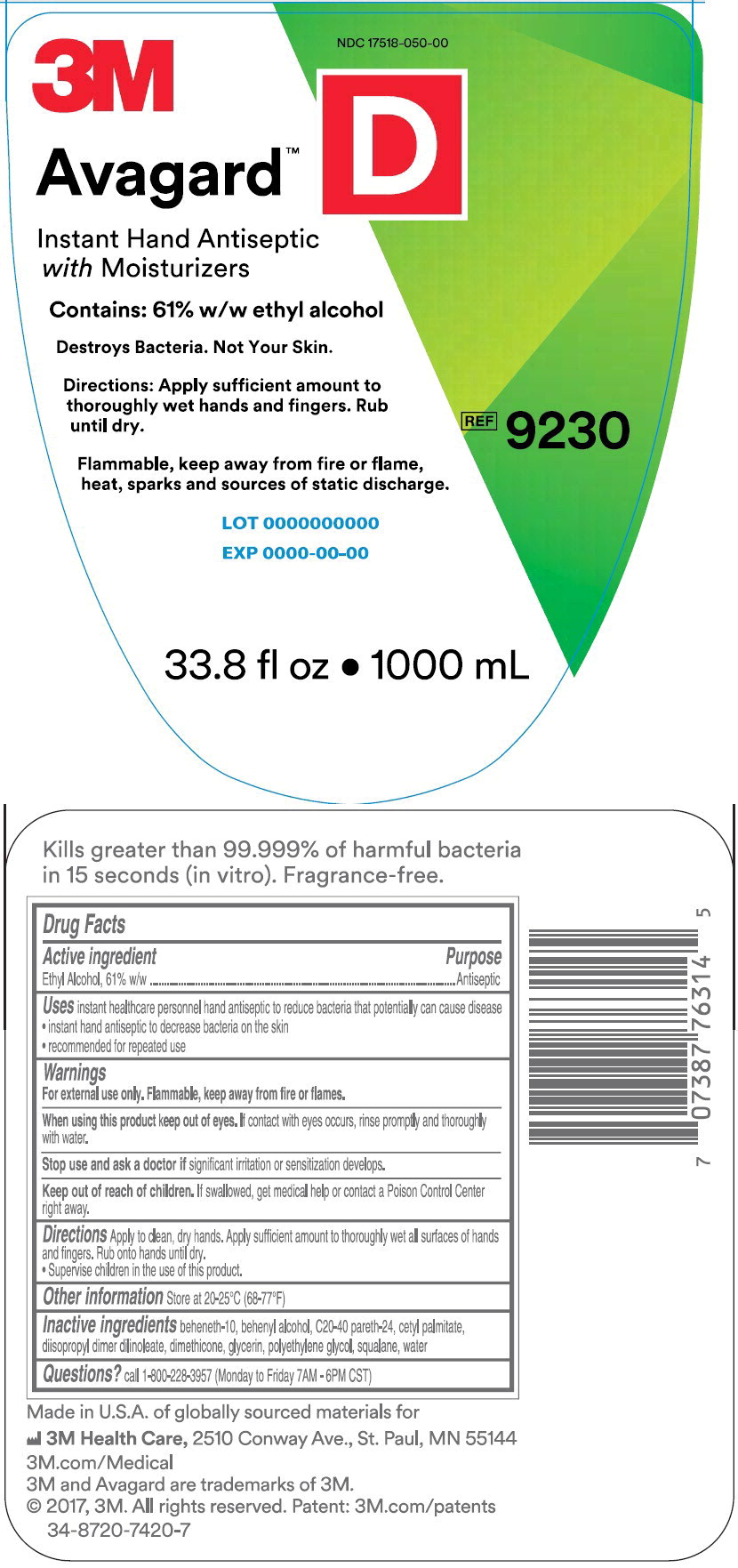
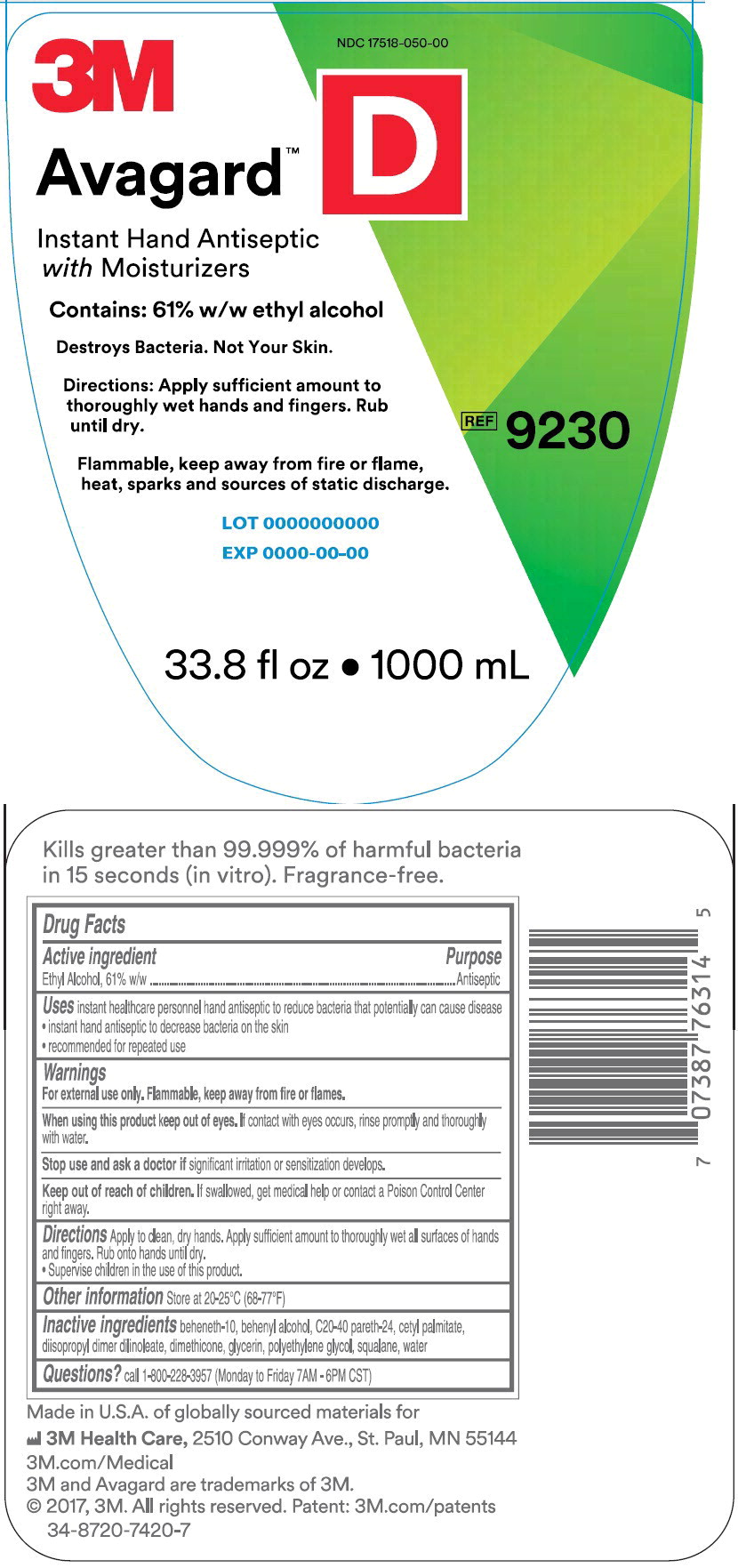
Principle Display Panel – 1000 mL Bottle Label
NDC 17518-050-00
3M
Avagard™ D
Instant Hand Antiseptic with Moisturizer
Contains: 61% w/w ethyl alcohol
Destroys Bacteria. Not Your Skin.
Directions: Apply sufficient amount to thoroughly wet hands and fingers.
Rub until dry.
Flammable, keep away from fire or flame, heat, sparks and sources of static discharge
REF 9230
33.8 fl oz • 1000 mL
-
INGREDIENTS AND APPEARANCE
3M AVAGARD D
alcohol lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17518-050 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Alcohol (UNII: 3K9958V90M) (Alcohol - UNII:3K9958V90M) Alcohol 530.7 mg in 1 mL Inactive Ingredients Ingredient Name Strength Beheneth-10 (UNII: 313S43DM16) docosanol (UNII: 9G1OE216XY) cetyl palmitate (UNII: 5ZA2S6B08X) dimethicone (UNII: 92RU3N3Y1O) glycerin (UNII: PDC6A3C0OX) Polyethylene Glycol, Unspecified (UNII: 3WJQ0SDW1A) squalane (UNII: GW89575KF9) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17518-050-01 88 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/1999 2 NDC:17518-050-02 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/1999 3 NDC:17518-050-00 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/1999 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 10/01/1999 Labeler - Solventum US OpCo LLC (006173082)