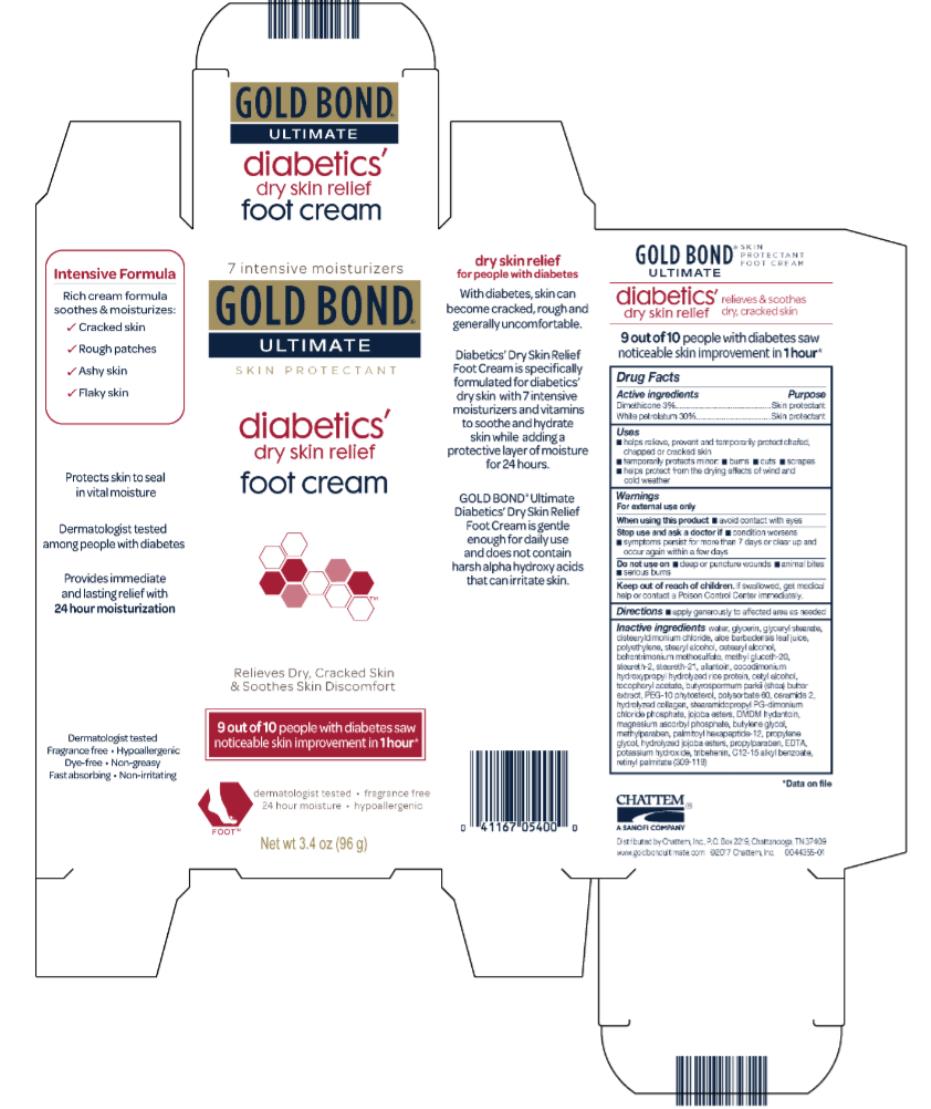
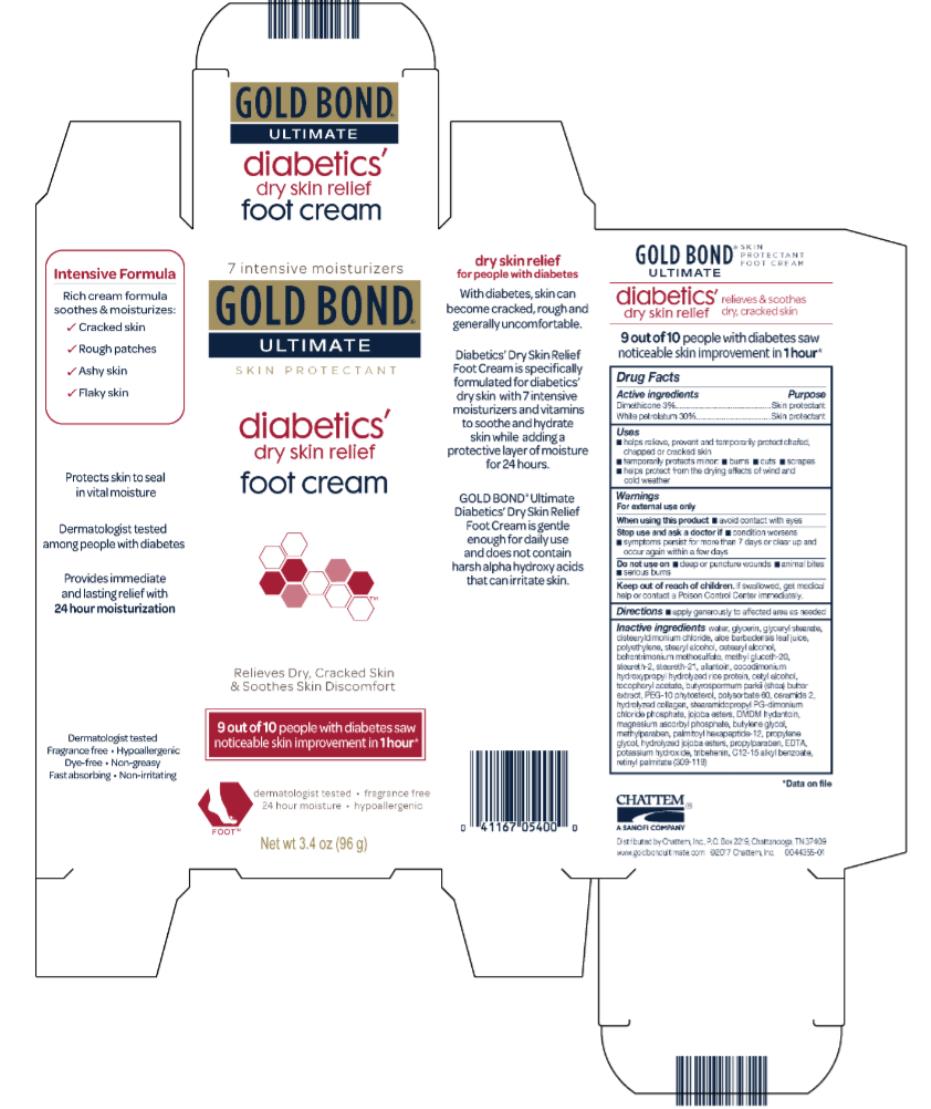
Label: GOLD BOND ULTIMATE DIABETICS- dimethicone and white petrolatum cream
- NDC Code(s): 41167-0540-0
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
water, glycerin, glyceryl stearate, distearyldimonium chloride, aloe barbadensis leaf juice, polyethylene, stearyl alcohol, cetearyl alcohol, methyl gluceth-20, behentrimonium methosulfate, steareth-21, steareth-2, allantoin, cocodimonium hydroxypropyl hydrolyzed rice protein, cetyl alcohol, tocopheryl acetate, butyrospermum parkii (shea) butter extract, PEG-10 rapeseed sterol, polysorbate 60, ceramide 2, hydrolyzed collagen, stearamidopropyl PG-dimonium chloride phosphate, jojoba esters, DMDM hydantoin, magnesium ascorbyl phosphate, butylenes glycol, methylparaben, palmitoyl oligopeptide, propylene glycol, hydrolyzed jojoba esters, propylparaben, EDTA, potassium hydroxide, tribehenin, C12-15 alkyl benzoate, retinyl palmitate (309-007)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GOLD BOND ULTIMATE DIABETICS
dimethicone and white petrolatum creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0540 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 0.03 g in 1 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 0.3 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) ALOE VERA LEAF (UNII: ZY81Z83H0X) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) METHYL GLUCETH-20 (UNII: J3QD0LD11P) BEHENTRIMONIUM METHOSULFATE (UNII: 5SHP745C61) STEARETH-21 (UNII: 53J3F32P58) STEARETH-2 (UNII: V56DFE46J5) ALLANTOIN (UNII: 344S277G0Z) CETYL ALCOHOL (UNII: 936JST6JCN) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SHEA BUTTER (UNII: K49155WL9Y) PEG-10 RAPESEED STEROL (UNII: 258O76T85M) POLYSORBATE 60 (UNII: CAL22UVI4M) CERAMIDE 2 (UNII: C04977SRJ5) STEARAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: W6000VEI5Y) DMDM HYDANTOIN (UNII: BYR0546TOW) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) METHYLPARABEN (UNII: A2I8C7HI9T) PALMITOYL HEXAPEPTIDE-12 (UNII: HO4ZT5S86C) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) HYDROLYZED JOJOBA ESTERS (ACID FORM) (UNII: UDR641JW8W) PROPYLPARABEN (UNII: Z8IX2SC1OH) EDETIC ACID (UNII: 9G34HU7RV0) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) TRIBEHENIN (UNII: 8OC9U7TQZ0) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0540-0 1 in 1 CARTON 02/03/1998 1 96 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 02/03/1998 Labeler - Chattem, Inc. (003336013)