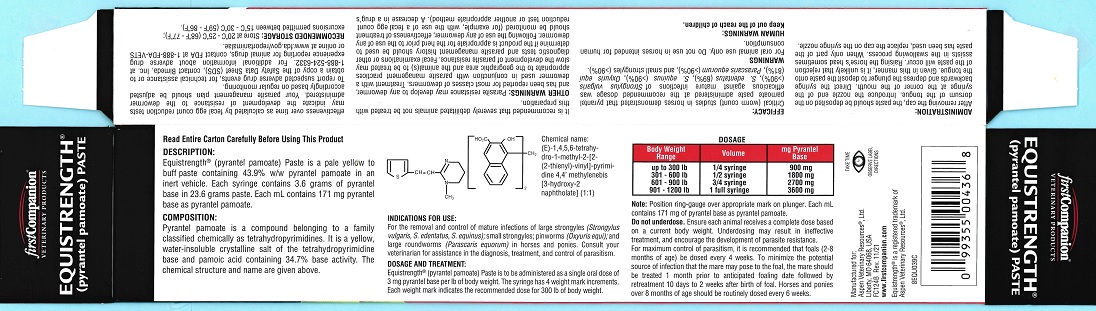
Label: EQUISTRENGTH- pyrantel pamoate paste
- NDC Code(s): 46066-912-01
- Packager: Aspen Veterinary
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Read Entire Carton Carefully Before Using This Product
DESCRIPTION:
Equistrength® Paste (pyrantel pamoate) is a pale yellow to buff paste containing 43.9% w/w pyrantel pamoate in an inert vehicle. Each syringe contains 3.6 grams pyrantel base in 23.6 grams paste. Each mL contains 171 mg pyrantel base as pyrantel pamoate.
-
COMPONENTS
COMPOSITION:
Pyrantel pamoate is a compound belonging to a family classified chemically as tetrahydropyrimidines. It is a yellow, water-insoluble crystalline salt of the tetrahydropyrimidine base and pamoic acid containing 34.7% base activity.
The chemical structure and name are given below:
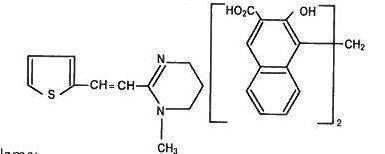
Chemical Name:
(E)-1,4,5,6-tetrahydro-1-methyl-2-[2-(2-thienyl)-vinyl]-pyrimidine 4,4' methylenebis [3-hydroxy-2 naphtholate] (1:1)
-
INDICATIONS & USAGE
INDICATIONS FOR USE:
For the removal and control of mature infections of large strongyles (Strongylus vulgaris, S. edentatus, S. equinus); small strongyles; pinworms (Oxyuris equi); and large roundworms (Parascaris equorum) in horses and ponies.
Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism.
-
DOSAGE & ADMINISTRATION
DOSAGE AND TREATMENT:
Equistrength® Paste (pyrantel pamoate) is to be administered as a single oral dose of 3 mg pyrantel base per lb of body weight. The syringe has four weight mark increments. Each weight mark indicates the recommended dose for 300 lb of body weight.
DOSAGE
Body Weight Range
Volume
mg Pyrantel Base
up to 300 lb
1/4 syringe
900 mg
301 - 600 lb
1/2 syringe
1800 mg
601 - 900 lb
3/4 syringe
2700 mg
901 - 1200 lb
1 full syringe
3600 mg
Note: Position ring-gauge over appropriate mark on plunger. Each mL contains 171 mg of pyrantel base as pyrantel pamoate.
Do not underdose. Ensure each animal receives a complete dose based on a current body weight. Underdosing may result in ineffective treatment, and encourage the development of parasite resistance.
For maximum control of parasitism, it is recommended that foals (2-8 months of age) be dosed every 4 weeks. To minimize the potential source of infection that the mare may pose to the foal, the mare should be treated 1 month prior to anticipated foaling date followed by re-treatment 10 days to 2 weeks after birth of foal. Horses and ponies over 8 months of age should be routinely dosed every 6 weeks.
ADMINISTRATION:
After removing the cap, the paste should be deposited on the dorsum of the tongue. Introduce the nozzle end of the syringe at the corner of the mouth. Direct the syringe backwards and depress the plunger to deposit the paste onto the tongue. Given in this manner, it is unlikely that rejection of the paste will occur. Raising the horse's head sometimes assists in the swallowing process. When only part of the paste has been used, replace the cap on the syringe nozzle.
- SUMMARY OF SAFETY AND EFFECTIVENESS
- WARNINGS
-
WARNINGS
OTHER WARNINGS: Parasite resistance may develop to any dewormer, and has been reported for most classes of dewormers. Treatment with a dewormer used in conjunction with parasite management practices appropriate to the geographic area and the animal(s) to be treated may slow the development of parasite resistance. Fecal examinations or other diagnostic tests and parasite management history should be used to determine if the product is appropriate for the herd prior to the use of any dewormer. Following the use of any dewormer, effectiveness of treatment should be monitored (for example, with the use of a fecal egg count reduction test or another appropriate method). A decrease in a drug's effectiveness over time as calculated by fecal egg count reduction tests may indicate the development of resistance to the dewormer administered. Your parasite management plan should be adjusted accordingly based on regular monitoring.
To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact Bimeda, Inc. at 1-888-524-6332. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EQUISTRENGTH
pyrantel pamoate pasteProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:46066-912 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRANTEL PAMOATE (UNII: 81BK194Z5M) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL 3.6 g in 23.6 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46066-912-01 23.6 g in 1 CARTON Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200350 01/19/2005 Labeler - Aspen Veterinary (627265361) Registrant - Bimeda, Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda-MTC 256232216 manufacture