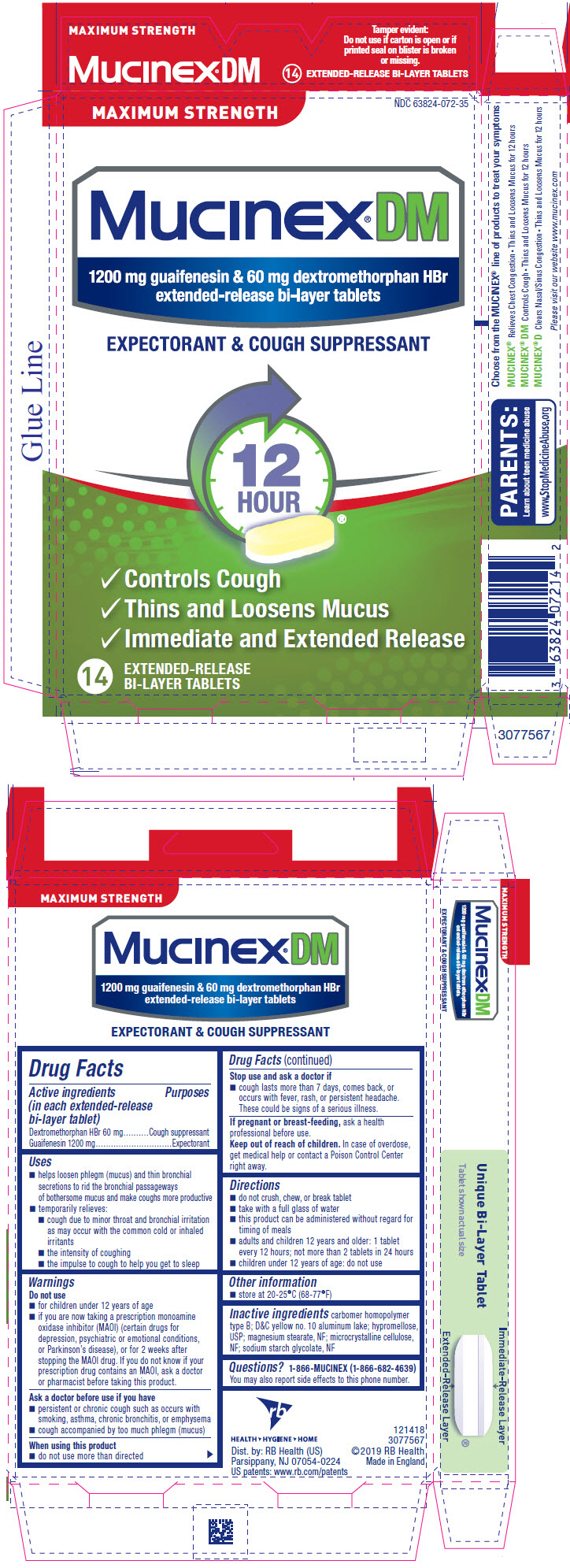
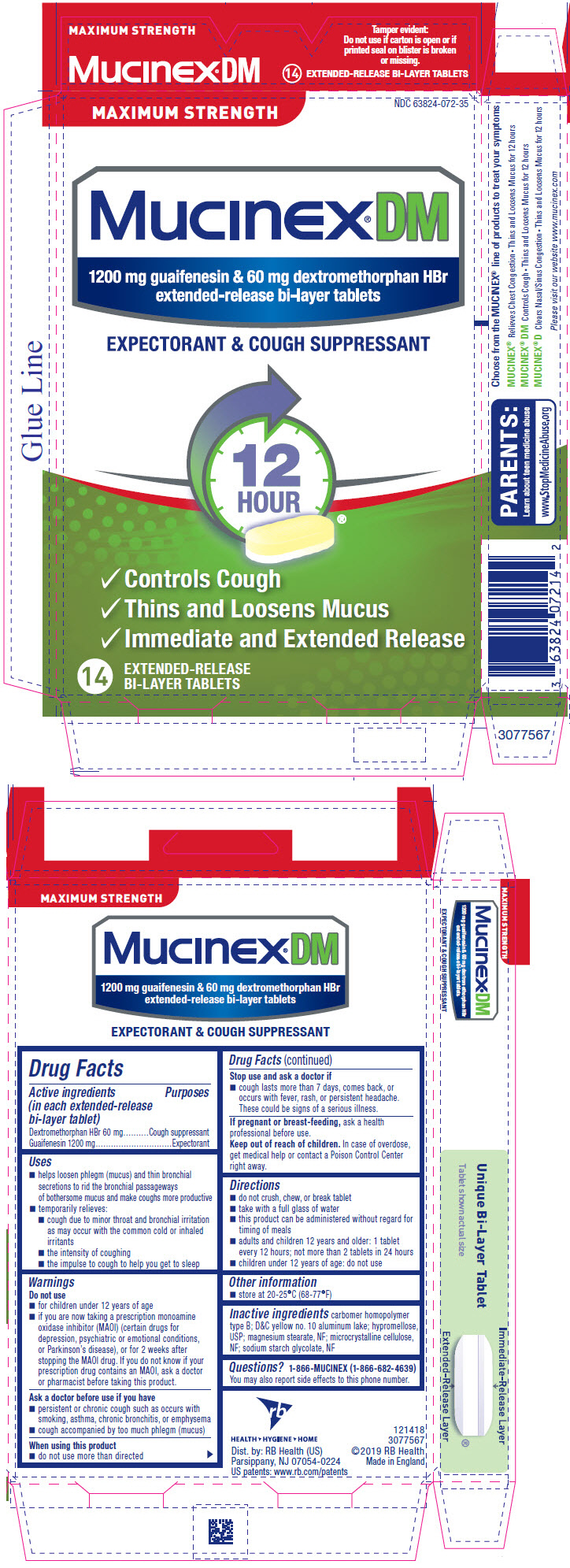
Label: MUCINEX DM MAXIMUM STRENGTH- guaifenesin and dextromethorphan hydrobromide tablet, extended release
-
NDC Code(s):
63824-072-02,
63824-072-07,
63824-072-18,
63824-072-35, view more63824-072-36, 63824-072-46, 63824-072-48, 63824-072-56
- Packager: RB Health (US) LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves:
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- the intensity of coughing
- the impulse to cough to help you get to sleep
-
Warnings
Do not use
- for children under 12 years of age
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 14 Tablet Blister Pack Carton
-
INGREDIENTS AND APPEARANCE
MUCINEX DM MAXIMUM STRENGTH
guaifenesin and dextromethorphan hydrobromide tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63824-072 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 1200 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 60 mg Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) ALUMINUM OXIDE (UNII: LMI26O6933) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color white (yellow and white) Score no score Shape OVAL Size 22mm Flavor Imprint Code Mucinex;1200 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63824-072-07 1 in 1 CARTON 06/26/2012 1 7 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:63824-072-35 1 in 1 CARTON 06/26/2012 2 14 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:63824-072-36 2 in 1 CARTON 06/26/2012 3 14 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:63824-072-46 3 in 1 CARTON 06/26/2012 4 14 in 1 BLISTER PACK; Type 0: Not a Combination Product 5 NDC:63824-072-48 4 in 1 CARTON 06/26/2012 09/01/2025 5 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 6 NDC:63824-072-18 2 in 1 CARTON 06/26/2012 06/15/2022 6 9 in 1 BLISTER PACK; Type 0: Not a Combination Product 7 NDC:63824-072-56 4 in 1 CARTON 07/01/2021 7 14 in 1 BLISTER PACK; Type 0: Not a Combination Product 8 NDC:63824-072-02 1 in 1 CARTON 08/01/2021 8 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021620 06/26/2012 Labeler - RB Health (US) LLC (081049410)