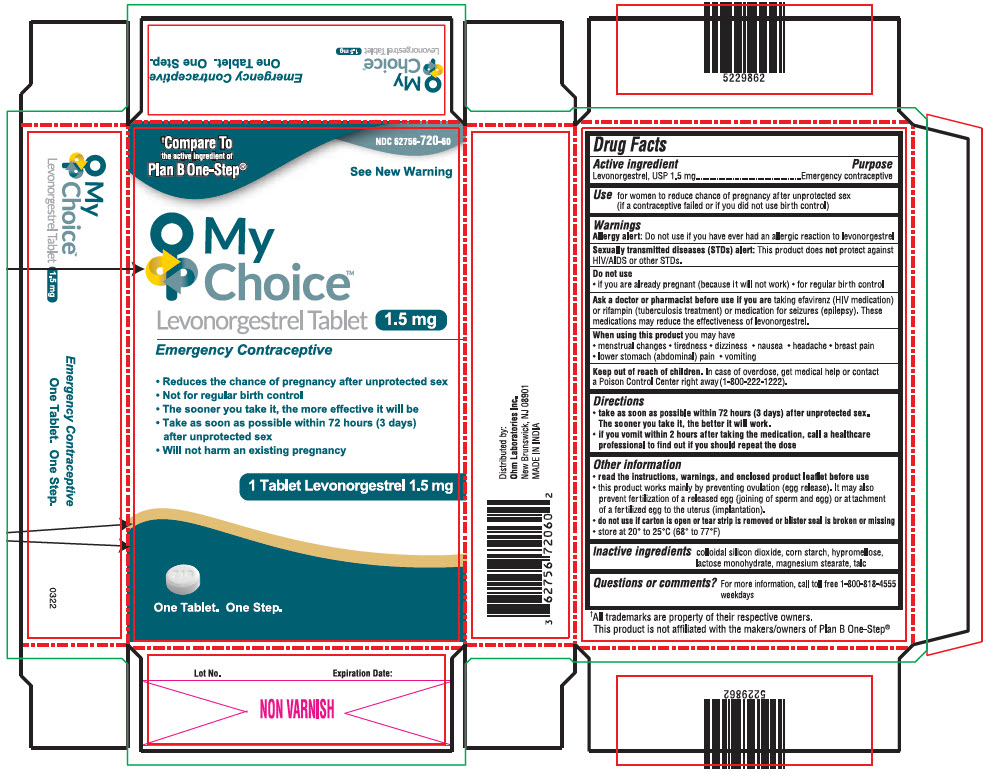
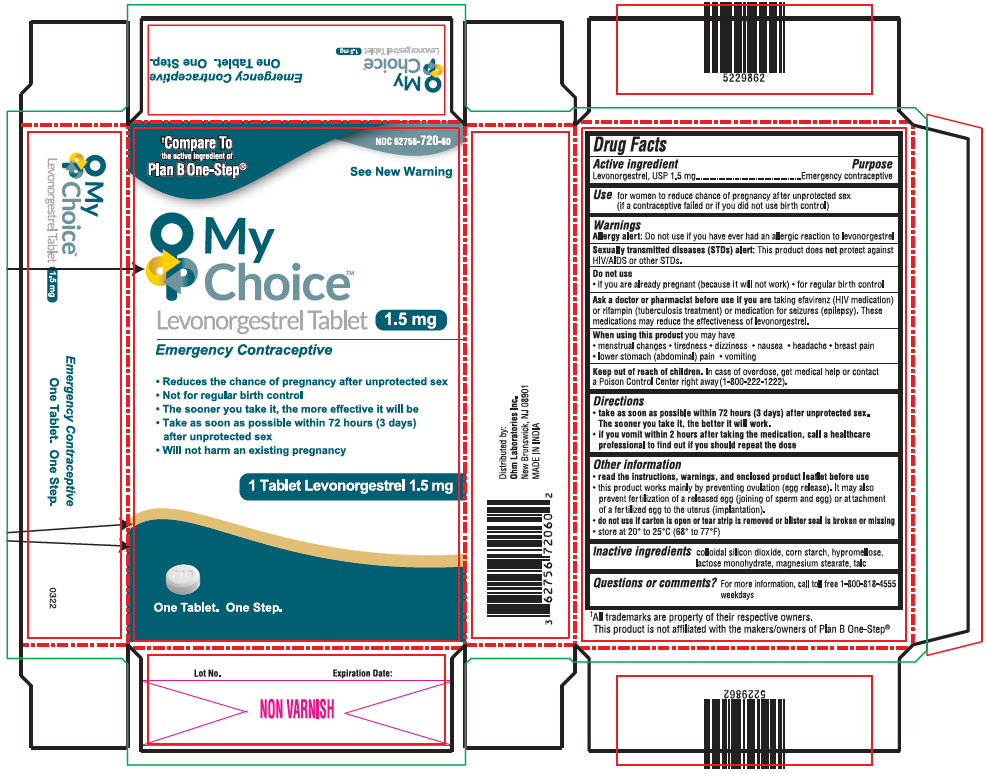
Label: MY CHOICE TM- levonorgestrel tablet
- NDC Code(s): 62756-720-60
- Packager: SUN PHARMACEUTICAL INDUSTRIES, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 21, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
Sexually transmitted diseases (STDs) alert
This product does not protect against HIV/AIDS or other STDs.
Ask a doctor or pharmacist before use if you are taking efavirenz (HIV medication) or rifampin (tuberculosis treatment) or medication for seizures (epilepsy). These medications may reduce the effectiveness of levonorgestrel.
- Directions
-
Other information
- •
- read the instructions, warnings, and enclosed product leaflet before use
- •
- this product works mainly by preventing ovulation (egg release). It may also prevent fer tilization of a released egg (joining of sperm and egg) or at tachment of a fer tilized egg to the uterus (implantation).
- •
- do not use if carton is open or tear strip is removed or blister seal is broken or missing
- •
- store at 20° to 25°C (68° to 77°F)
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 1.5 mg Tablet Blister Pack Carton
NDC 62756-720-60
†Compare To
the active ingredient of
Plan B One-Step®See New Warning
My
Choice™
Levonorgestrel Tablet 1.5 mg
Emergency Contraceptive- Reduces the chance of pregnancy after unprotected sex
- Not for regular birth control
- The sooner you take it, the more effective it will be
- Take as soon as possible within 72 hours (3 days)
after unprotected sex - Will not harm an existing pregnancy
1 Tablet Levonorgestrel 1.5 mg
One Tablet. One Step.
-
INGREDIENTS AND APPEARANCE
MY CHOICE TM
levonorgestrel tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62756-720 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVONORGESTREL (UNII: 5W7SIA7YZW) (LEVONORGESTREL - UNII:5W7SIA7YZW) LEVONORGESTREL 1.5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND (circular) Size 8mm Flavor Imprint Code 718 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62756-720-60 1 in 1 CARTON 04/01/2018 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202635 04/01/2018 Labeler - SUN PHARMACEUTICAL INDUSTRIES, INC. (146974886) Establishment Name Address ID/FEI Business Operations Sun Pharmaceutical Industries Limited 725959238 ANALYSIS(62756-720) , MANUFACTURE(62756-720)