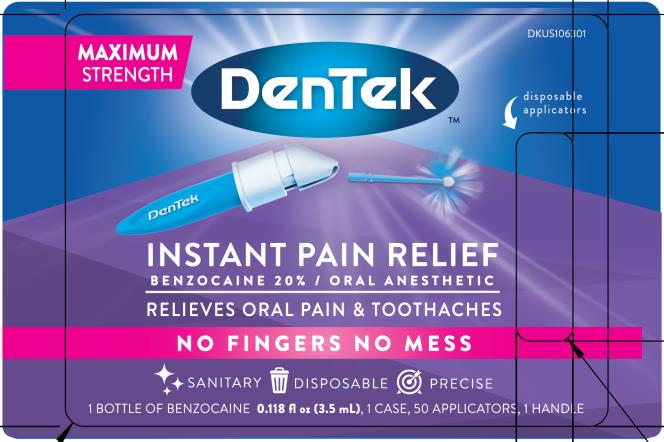
Label: DENTEK INSTANT PAIN RELIEF MAXIMUM STRENGTH- benzocaine liquid
- NDC Code(s): 60630-077-03, 60630-077-04
- Packager: DenTek Oral Care, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 18, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
METHEMOGLOBINEMIA WARNING: Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
- pale, gray, or blue colored skin (cyanosis)
- headache
- rapid heart rate
- shortness of breath
- dizziness or lightheadedness
- fatigue or lack of energy
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other “caine” anesthetics.
When using this product
- avoid contact with the eyes
- do not exceed recommended dosage
- do not use for more than 7 days unless directed by a doctor/dentist
- pale, gray, or blue colored skin (cyanosis)
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DENTEK INSTANT PAIN RELIEF MAXIMUM STRENGTH
benzocaine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60630-077 Route of Administration DENTAL, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength PEPPERMINT OIL (UNII: AV092KU4JH) 2.2 mg in 1 mL POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) 784.5 mg in 1 mL SPEARMINT OIL (UNII: C3M81465G5) 3.3 mg in 1 mL SUCRALOSE (UNII: 96K6UQ3ZD4) 10 mg in 1 mL Product Characteristics Color yellow (colorless to light yellow liquid) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60630-077-04 1 in 1 CARTON 07/31/2015 1 NDC:60630-077-03 3.5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 07/31/2015 Labeler - DenTek Oral Care, Inc. (153818646) Registrant - DenTek Oral Care, Inc. (153818646)