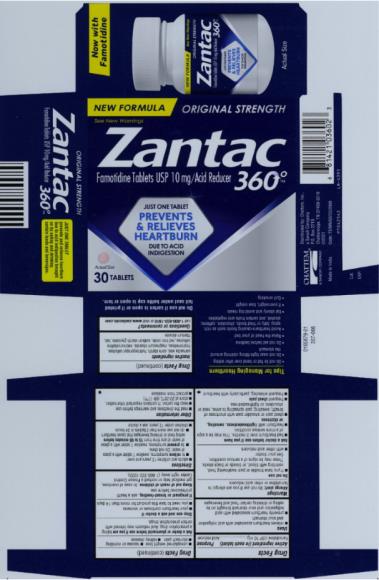
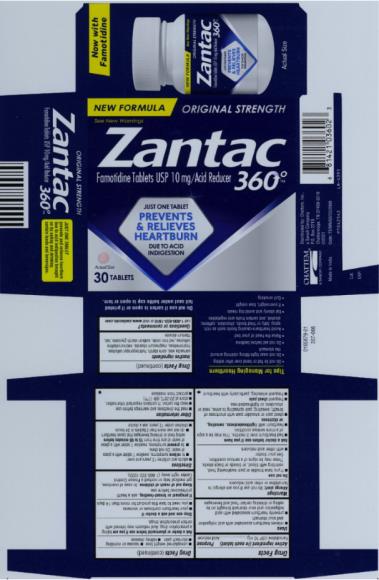
Label: ZANTAC 360- famotidine tablet, film coated
-
NDC Code(s):
41167-0360-2,
41167-0361-0,
41167-0361-1,
41167-0361-2, view more41167-0361-4, 41167-0361-6, 41167-0361-8, 41167-0361-9
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 17, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Allergy alert: Do not use if you are allergic to famotidine or other acid reducers
Do not use
■ if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
■ with other acid reducers
Ask a doctor before use if you have
■ had heartburn over 3 months. This may be a sign of a more serious condition.
■ heartburn with lightheadedness, sweating or dizziness
■ chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
■ frequent chest pain
■ frequent wheezing, particularly with heartburn
■ unexplained weight loss
■ nausea or vomiting
■ stomach pain
■ kidney disease
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Acid reducers may interact with certain prescription drugs.
-
Directions
■ adults and children 12 years and over:
■ to relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
■ to prevent symptoms, swallow 1 tablet with a glass of water at any time from 15 to 60 minutes before eating food or drinking beverages that cause heartburn
■ do not use more than 2 tablets in 24 hours
■ children under 12 years: ask a doctor
- Other information
- Inactive ingredients
-
Questions or comments
call 1-800-633-1610 or visit www.zantacotc.com
Tips for Managing Heartburn
■ Do not lie flat or bend over after eating
■ Do not wear tight-fitting clothing around the stomach
■ Do not eat before bedtime
■ Raise the head of your bed
■ Avoid heartburn-causing foods such as rich, spicy, fatty or fried foods, chocolate, caffeine, alcohol, and certain fruits and vegetables
■ Eat slowly and avoid big meals
■ If overweight, lose weight
■ Quit smoking
Zantac 360⁰
Famotidine Tablets 20 mg / Acid Reducer
Drug Facts
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Allergy alert: Do not use if you are allergic to famotidine or other acid reducers
Do not use
■ if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
■ with other acid reducers
Ask a doctor before use if you have
■ had heartburn over 3 months. This may be a sign of a more serious condition.
■ heartburn with lightheadedness, sweating or dizziness
■ chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
■ frequent chest pain
■ frequent wheezing, particularly with heartburn
■ unexplained weight loss
■ nausea or vomiting
■ stomach pain
■ kidney disease
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Acid reducers may interact with certain prescription drugs.
-
Directions
■ adults and children 12 years and over:
■ to relieve symptoms, swallow 1 tablet with a glass of water. Do not chew.
■ to prevent symptoms, swallow 1 tablet with a glass of water at any time from 10 to 60 minutes before eating food or drinking beverages that cause heartburn
■ do not use more than 2 tablets in 24 hours
■ children under 12 years: ask a doctor
- Other information
- Inactive ingredients
-
Questions or comments?
call 1-800-633-1610 or visit www.zantacotc.com
Tips for Managing Heartburn
■ Do not lie flat or bend over after eating
■ Do not wear tight-fitting clothing around the stomach
■ Do not eat before bedtime
■ Raise the head of your bed
■ Avoid heartburn-causing foods such as rich, spicy, fatty or fried foods, chocolate, caffeine, alcohol, and certain fruits and vegetables
■ Eat slowly and avoid big meals
■ If overweight, lose weight
■ Quit smoking
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ZANTAC 360
famotidine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0360 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FAMOTIDINE (UNII: 5QZO15J2Z8) (FAMOTIDINE - UNII:5QZO15J2Z8) FAMOTIDINE 10 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) FERRIC OXIDE RED (UNII: 1K09F3G675) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) Product Characteristics Color pink Score no score Shape ROUND (biconvex) Size 5mm Flavor Imprint Code CC;58 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0360-2 1 in 1 CARTON 04/12/2021 1 30 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206531 04/12/2021 ZANTAC 360
famotidine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0361 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FAMOTIDINE (UNII: 5QZO15J2Z8) (FAMOTIDINE - UNII:5QZO15J2Z8) FAMOTIDINE 20 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) FERRIC OXIDE RED (UNII: 1K09F3G675) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) Product Characteristics Color yellow Score no score Shape ROUND (Square shaped Biconvex) Size 5mm Flavor Imprint Code CC;59 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0361-0 1 in 1 CARTON 04/12/2021 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:41167-0361-2 1 in 1 CARTON 04/12/2021 2 25 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:41167-0361-4 1 in 1 CARTON 04/12/2021 3 50 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:41167-0361-9 2 in 1 CELLO PACK 04/12/2021 4 70 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:41167-0361-6 1 in 1 CARTON 04/12/2021 5 90 in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC:41167-0361-8 1 in 1 CARTON 04/12/2021 6 100 in 1 BOTTLE; Type 0: Not a Combination Product 7 NDC:41167-0361-1 1 in 1 CARTON 04/12/2021 7 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206531 04/12/2021 Labeler - Chattem, Inc. (003336013)