Label: DEXMETHYLPHENIDATE HYDROCHLORIDE tablet
- NDC Code(s): 27808-091-01, 27808-092-01, 27808-093-01
- Packager: Tris Pharma Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DEXMETHYLPHENIDATE HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for DEXMETHYLPHENIDATE HYDROCHLORIDE TABLETS.
DEXMETHYLPHENIDATE HYDROCHLORIDE tablets, for oral use, CII
Initial U.S. Approval: 2001
WARNING: ABUSE, MISUSE, AND ADDICTION
See full prescribing information for complete boxed warning.
Dexmethylphenidate hydrochloride has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction.
Misuse and abuse of CNS stimulants, including dexmethylphenidate hydrochloride tablets, can result in overdose and death (5.1, 9.2, 10):
- Before prescribing dexmethylphenidate hydrochloride tablets, assess each patient’s risk for abuse, misuse, and addiction.
- Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug.
- Throughout treatment, reassess each patient’s risk and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Dexmethylphenidate hydrochloride tablets are a central nervous system (CNS) stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) (1).
DOSAGE AND ADMINISTRATION
- Administer orally twice daily, 4 hours apart with or without food (2).
- For patients new to methylphenidate: Recommend starting dose of 5 mg once daily (2.5 mg twice daily) (2.2).
- For patients currently taking methylphenidate: Initiate dexmethylphenidate hydrochloride tablets therapy with half (1/2) the current total daily dose of methylphenidate (2.2).
- Titrate weekly in increments of 2.5 to 5 mg to a maximum of 20 mg/day (10 mg twice daily) (2.2).
DOSAGE FORMS AND STRENGTHS
Tablets: 2.5 mg, 5 mg, and 10 mg (3).
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Risks to Patients with Serious Cardiac Disease: Avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious cardiac arrhythmias, coronary artery disease, or other serious cardiac disease (5.2).
- Increased Blood Pressure and Heart Rate: Monitor blood pressure and pulse (5.3).
- Psychiatric Adverse Reactions: Prior to initiating dexmethylphenidate hydrochloride tablets, screen patients for risk factors for developing a manic episode. If new psychotic or manic symptoms occur, consider discontinuing dexmethylphenidate hydrochloride tablets (5.4).
- Priapism: If abnormally sustained or frequent and painful erections occur, patients should seek immediate medical attention (5.5).
- Peripheral Vasculopathy, Including Raynaud’s Phenomenon: Careful observation for digital changes is necessary during dexmethylphenidate hydrochloride treatment. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for patients who develop signs or symptoms of peripheral vasculopathy (5.6).
- Long-Term Suppression of Growth in Pediatric Patients: Closely monitor growth (height and weight) in pediatric patients. Pediatric patients not growing or gaining height or weight as expected may need to have their treatment interrupted (5.7).
- Acute Angle Closure Glaucoma: Dexmethylphenidate hydrochloride -treated patients considered at risk for acute angle closure glaucoma (e.g., patients with significant hyperopia) should be evaluated by an ophthalmologist (5.8).
- Increased Intraocular Pressure (IOP) and Glaucoma: Prescribe dexmethylphenidate hydrochloride to patients with open-angle glaucoma or abnormally increased IOP only if the benefit of treatment is considered to outweigh the risk. Closely monitor patients with a history of increased IOP or open angle glaucoma (5.9).
- Motor and Verbal Tics, and Worsening of Tourette’s Syndrome: Before initiating dexmethylphenidate hydrochloride, assess the family history and clinically evaluate patients for tics or Tourette’s syndrome. Regularly monitor patients for the emergence or worsening of tics or Tourette’s syndrome. Discontinue treatment if clinically appropriate (5.10).
ADVERSE REACTIONS
The most common adverse reactions (greater than or equal to 5% and twice the rate of placebo) in pediatric patients 6 to 17 years were abdominal pain, fever, nausea, and anorexia (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Tris Pharma, Inc. at 1-732-940-0358 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
- Antihypertensive Drugs: Monitor blood pressure. Adjust dosage of antihypertensive drug as needed (7.1).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: ABUSE, MISUSE, AND ADDICTION
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Pretreatment Screening
2.2 Recommended Dosage
2.3 Administration Instructions
2.4 Dosage Reduction and Discontinuation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Abuse, Misuse, and Addiction
5.2 Risks to Patients With Serious Cardiac Disease
5.3 Increased Blood Pressure and Heart Rate
5.4 Psychiatric Adverse Reactions
5.5 Priapism
5.6 Peripheral Vasculopathy, Including Raynaud’s Phenomenon
5.7 Long-Term Suppression of Growth in Pediatric Patients
5.8 Acute Angle Closure Glaucoma
5.9 Increased Intraocular Pressure and Glaucoma
5.10 Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Clinically Important Drug Interactions with Dexmethylphenidate Hydrochloride Tablets
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: ABUSE, MISUSE, AND ADDICTION
Dexmethylphenidate hydrochloride has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including dexmethylphenidate hydrochloride, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing dexmethylphenidate hydrochloride, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout dexmethylphenidate hydrochloride treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction [see Warnings and Precautions (5.1) and Drug Abuse and Dependence (9.2)].
-
1 INDICATIONS AND USAGE
Dexmethylphenidate hydrochloride tablets are indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Pretreatment Screening
Prior to treating patients with dexmethylphenidate hydrochloride tablets, assess:
- for the presence of cardiac disease (i.e., perform a careful history, family history of sudden death or ventricular arrhythmia, and physical exam) [see Warnings and Precautions (5.2)].
- the family history and clinically evaluate patients for motor or verbal tics or Tourette’s syndrome before initiating dexmethylphenidate hydrochloride tablets [see Warnings and Precautions (5.10)].
2.2 Recommended Dosage
Patients New to Methylphenidate
The recommended starting dose of dexmethylphenidate hydrochloride tablets for pediatric patients who are not currently taking racemic methylphenidate, or for patients who are on stimulants other than methylphenidate, is 5 mg daily (2.5 mg twice daily) with or without food.
Patients Currently on Methylphenidate
The recommended starting dose of dexmethylphenidate hydrochloride tablets for pediatric patients currently using methylphenidate is half (1/2) the total daily dose of racemic methylphenidate.
Titration Schedule
The dose may be titrated weekly in increments of 2.5 mg to 5 mg to a maximum of 20 mg daily (10 mg twice daily). The dose should be individualized according to the needs and response of the patient.
2.3 Administration Instructions
Dexmethylphenidate hydrochloride tablets are administered orally twice daily, at least 4 hours apart.
2.4 Dosage Reduction and Discontinuation
If paradoxical aggravation of symptoms or other adverse reactions occur, reduce the dosage, or if necessary, discontinue dexmethylphenidate hydrochloride tablets. If improvement is not observed after appropriate dosage adjustment over a one-month period, the drug should be discontinued.
-
3 DOSAGE FORMS AND STRENGTHS
Dexmethylphenidate Hydrochloride Tablets, 2.5 mg are blue, round-shaped, convex tablets debossed with 91 on one side and plain on the other side
Dexmethylphenidate Hydrochloride Tablets, 5 mg are yellow, round-shaped, convex tablets debossed with 92 on one side and plain on the other side
Dexmethylphenidate Hydrochloride Tablets, 10 mg are white to off white, round-shaped, convex tablets debossed with 93 on one side and plain on the other side
-
4 CONTRAINDICATIONS
- Hypersensitivity to methylphenidate or other components of dexmethylphenidate hydrochloride tablets. Hypersensitivity reactions, such as angioedema and anaphylactic reactions have been reported in patients treated with methylphenidate [see Adverse Reactions (6.1)].
- Concomitant treatment with monoamine oxidase inhibitors (MAOIs), or within 14 days following discontinuation of treatment with an MAOI, because of the risk of hypertensive crises [see Drug Interactions (7.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Abuse, Misuse, and Addiction
Dexmethylphenidate hydrochloride has a high potential for abuse and misuse. The use of dexmethylphenidate hydrochloride exposes individuals to the risks of abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Dexmethylphenidate hydrochloride tablets can be diverted for non-medical use into illicit channels or distribution [see Drug Abuse and Dependence (9.2)]. Misuse and abuse of CNS stimulants, including dexmethylphenidate hydrochloride tablets, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing dexmethylphenidate hydrochloride tablets, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks and proper disposal of any unused drug. Advise patients to store dexmethylphenidate hydrochloride in a safe place, preferably locked, and instruct patients to not give dexmethylphenidate hydrochloride tablets to anyone else. Throughout dexmethylphenidate hydrochloride treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
5.2 Risks to Patients With Serious Cardiac Disease
Sudden death has been reported in patients with structural cardiac abnormalities or other serious cardiac disease who were treated with CNS stimulants at the recommended ADHD dosage.
Avoid dexmethylphenidate hydrochloride use in patients with known structural cardiac abnormalities, cardiomyopathy, serious cardiac arrhythmia, coronary artery disease, or other serious cardiac disease.
5.3 Increased Blood Pressure and Heart Rate
CNS stimulants cause an increase in blood pressure (mean increase approximately 2 to 4 mmHg) and heart rate (mean increase approximately 3 to 6 beats per minute). Some patients may have larger increases.
Monitor all dexmethylphenidate hydrochloride-treated patients for hypertension and tachycardia.
5.4 Psychiatric Adverse Reactions
Exacerbation of Preexisting Psychosis
CNS stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a preexisting psychotic disorder.
Induction of a Manic Episode in Patients with Bipolar Disorder
CNS stimulants may induce a manic or mixed mood episode in patients. Prior to initiating treatment, screen patients for risk factors for developing a manic episode (e.g., comorbid or history of depressive symptoms or a family history of suicide, bipolar disorder, or depression).
New Psychotic or Manic Symptoms
CNS stimulants, at the recommended dosage, may cause psychotic or manic symptoms (e.g., hallucinations, delusional thinking, or mania) in patients without a prior history of psychotic illness or mania. In a pooled analysis of multiple short-term, placebo-controlled studies of CNS stimulants, psychotic, or manic symptoms occurred in approximately 0.1% of CNS stimulant-treated patients, compared to 0% of placebo-treated patients. If such symptoms occur, consider discontinuing dexmethylphenidate hydrochloride tablets.
5.5 Priapism
Prolonged and painful erections, sometimes requiring surgical intervention, have been reported with methylphenidate use in both adult and pediatric male patients. Although priapism was not reported with methylphenidate initiation, it developed after some time on methylphenidate, often subsequent to an increase in dosage. Priapism has also occurred during methylphenidate withdrawal (drug holidays or during discontinuation).
Dexmethylphenidate hydrochloride-treated patients who develop abnormally sustained or frequent and painful erections should seek immediate medical attention.
5.6 Peripheral Vasculopathy, Including Raynaud’s Phenomenon
CNS stimulants, including dexmethylphenidate hydrochloride tablets, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud’s phenomenon. Signs and symptoms are usually intermittent and mild; however, sequelae have included digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud’s phenomenon, were observed in post-marketing reports and at the therapeutic dosages of CNS stimulants in all age groups throughout the course of treatment. Signs and symptoms generally improved after dosage reduction or discontinuation of the CNS stimulant.
Careful observation for digital changes is necessary during dexmethylphenidate hydrochloride treatment. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for dexmethylphenidate hydrochloride-treated patients who develop signs or symptoms of peripheral vasculopathy.
5.7 Long-Term Suppression of Growth in Pediatric Patients
CNS stimulants have been associated with weight loss and slowing of growth rate in pediatric patients.
Careful follow-up of weight and height in patients ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated patients over 36 months (to the ages of 10 to 13 years), suggests that pediatric patients who received methylphenidate for 7 days per week throughout the year had a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this development period.
Closely monitor growth (weight and height) in dexmethylphenidate hydrochloride-treated pediatric patients. Pediatric patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted.
5.8 Acute Angle Closure Glaucoma
There have been reports of angle closure glaucoma associated with methylphenidate treatment.
Although the mechanism is not clear, dexmethylphenidate hydrochloride-treated patients considered at risk for acute angle closure glaucoma (e.g., patients with significant hyperopia) should be evaluated by an ophthalmologist.
5.9 Increased Intraocular Pressure and Glaucoma
There have been reports of an elevation of intraocular pressure (IOP) associated with methylphenidate treatment [see Adverse Reactions (6.2)].
Prescribe dexmethylphenidate hydrochloride tablets to patients with open-angle glaucoma or abnormally increased IOP only if the benefit of treatment is considered to outweigh the risk. Closely monitor dexmethylphenidate hydrochloride-treated patients with a history of abnormally increased IOP or open angle glaucoma.
5.10 Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
CNS stimulants, including methylphenidate, have been associated with the onset or exacerbation of motor and verbal tics. Worsening of Tourette’s syndrome has also been reported [see Adverse Reactions (6.2)].
Before initiating dexmethylphenidate hydrochloride, assess the family history and clinically evaluate patients for tics or Tourette’s syndrome. Regularly monitor dexmethylphenidate hydrochloride-treated patients for the emergence or worsening of tics or Tourette’s syndrome, and discontinue treatment if clinically appropriate.
-
6 ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
- Abuse, Misuse, and Addiction [see Boxed Warning, Warnings and Precautions (5.1), Drug Abuse and Dependence (9.2, 9.3)]
- Known hypersensitivity to methylphenidate or other ingredients of dexmethylphenidate hydrochloride tablets [see Contraindications (4)]
- Hypertensive crisis with Concomitant Use of Monoamine Oxidase Inhibitors [see Contraindications (4), Drug Interactions (7.1)]
- Risks to Patients with Serious Cardiac Disease [see Warnings and Precautions (5.2)]
- Increased Blood Pressure and Heart Rate [see Warnings and Precautions (5.3)]
- Psychiatric Adverse Reactions [see Warnings and Precautions (5.4)]
- Priapism [see Warnings and Precautions (5.5)]
- Peripheral Vasculopathy, Including Raynaud’s phenomenon [see Warnings and Precautions (5.6)]
- Long-Term Suppression of Growth in Pediatric Patients [see Warnings and Precautions (5.7)]
- Acute Angle Closure Glaucoma [see Warnings and Precautions (5.8)]
- Increased Intraocular Pressure and Glaucoma [see Warnings and Precautions (5.9)]
- Motor and Verbal Tics, and Worsening of Tourette’s Syndrome [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adverse Reactions in Studies with Dexmethylphenidate Hydrochloride Tablets in Pediatric Patients with ADHD
The safety data in this section is based on data related to dexmethylphenidate hydrochloride tablets exposure during the premarketing development program in a total of 696 participants in clinical trials (684 patients, 12 healthy adult subjects). These participants received dexmethylphenidate hydrochloride tablets 5, 10, or 20 mg/day. The 684 ADHD patients (ages 6 to 17 years) were evaluated in 2 controlled clinical studies, 2 clinical pharmacology studies, and 2 open-label long-term safety studies.
Most Common Adverse Reactions (incidence of greater than or equal to 5% and at least twice placebo): abdominal pain, fever, anorexia, and nausea
Adverse Reactions Leading to Discontinuation: Overall, 50 of 684 (7.3%) pediatric patients treated with dexmethylphenidate hydrochloride tablets experienced an adverse reaction that resulted in discontinuation. The most common reasons for discontinuation were twitching (described as motor or vocal tics), anorexia, insomnia, and tachycardia (approximately 1% each).
Table 1 enumerates adverse reactions for two, placebo-controlled, parallel group studies in pediatric patients with ADHD taking dexmethylphenidate hydrochloride tablets doses of 5, 10, and 20 mg/day. The table includes only those reactions that occurred in patients treated with dexmethylphenidate hydrochloride tablets for which the incidence was at least 5% and twice the incidence among placebo-treated patients.
Table 1: Common Adverse Reactions in Pediatric Patients (6 to 17 years of age) With ADHD System organ class Adverse reactions Dexmethylphenidate Hydrochloride Tablets
(N = 79)Placebo
(N = 82)Body as a whole Abdominal pain 15% 6% Fever 5% 1% Digestive system Anorexia 6% 1% Nausea 9% 1% Abbreviation: ADHD, attention deficit hyperactivity disorder.
6.2 Postmarketing Experience
The following additional adverse reactions have been identified during postapproval use of dexmethylphenidate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Musculoskeletal: rhabdomyolysis
Immune System Disorders: hypersensitivity reactions, such as angioedema, anaphylactic reactions
Adverse Reactions Reported with All Methylphenidate Hydrochloride and Dexmethylphenidate Hydrochloride Tablets Formulations
The following adverse reactions associated with the use of all methylphenidate hydrochloride and dexmethylphenidate hydrochloride formulations were identified in clinical trials, spontaneous reports, and literature. Because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Infections and Infestations: nasopharyngitis
Blood and the Lymphatic System Disorders: leukopenia, thrombocytopenia, anemia
Immune System Disorders: hypersensitivity reactions, including angioedema and anaphylaxis
Metabolism and Nutrition Disorders: decreased appetite, reduced weight gain, and suppression of growth during prolonged use in pediatric patients
Psychiatric Disorders: insomnia, anxiety, restlessness, agitation, psychosis (sometimes with visual and tactile hallucinations), depressed mood, depression
Nervous System Disorders: headache, dizziness, tremor, dyskinesia, including choreoatheetoid movements, drowsiness, convulsions, cerebrovascular disorders (including vasculitis, cerebral hemorrhages, and cerebrovascular accidents), serotonin syndrome in combination with serotonergic drugs
Eye Disorders: blurred vision, difficulties in visual accommodation
Cardiac Disorders: tachycardia, palpitations, increased blood pressure, arrhythmias, angina pectoris
Respiratory, Thoracic, and Mediastinal Disorders: cough
Gastrointestinal Disorders: dry mouth, nausea, vomiting, abdominal pain, dyspepsia
Hepatobiliary Disorders: abnormal liver function, ranging from transaminase elevation to severe hepatic injury
Skin and Subcutaneous Tissue Disorders: hyperhidrosis, pruritus, urticaria, exfoliative dermatitis, scalp hair loss, erythema multiforme rash, thrombocytopenic purpura
Musculoskeletal and Connective Tissue Disorders: arthralgia, muscle cramps, rhabdomyolysis, trismus
Investigations: weight loss (adult ADHD patients)
Vascular Disorders: peripheral coldness, Raynaud's phenomenon
Additional Adverse Reactions Reported with Other Methylphenidate-Containing Products
The list below shows adverse reactions not listed with methylphenidate hydrochloride and dexmethylphenidate hydrochloride formulations that have been reported with other methylphenidate products based on clinical trials data and post-marketing spontaneous reports.
Blood and Lymphatic Disorders: pancytopenia
Immune System Disorders: hypersensitivity reactions, such as auricular swelling
Psychiatric Disorders: affect lability, mania, disorientation, libido changes
Nervous System Disorders: migraine, motor and verbal Tics
Eye Disorders: diplopia, increased intraocular pressure, mydriasis
Cardiac Disorders: sudden cardiac death, myocardial infarction, bradycardia, extrasystole, supraventricular tachycardia, ventricular extrasystole
Respiratory, Thoracic, and Mediastinal Disorders: pharyngolaryngeal pain, dyspnea
Gastrointestinal Disorders: diarrhea, constipation
Skin and Subcutaneous Tissue Disorders: angioneurotic edema, erythema, fixed drug eruption
Musculoskeletal, Connective Tissue, and Bone Disorders: myalgia, muscle twitching
Renal and Urinary Disorders: hematuria
Reproductive System and Breast Disorders: gynecomastia
General Disorders: fatigue
Urogenital Disorders: priapism
-
7 DRUG INTERACTIONS
7.1 Clinically Important Drug Interactions with Dexmethylphenidate Hydrochloride Tablets
Table 2 presents clinically important drug interactions with dexmethylphenidate hydrochloride tablets.
Table 2: Clinically Important Drug Interactions with Dexmethylphenidate Hydrochloride Tablets Monoamine Oxidase Inhibitors (MAOIs) Clinical impact Concomitant use of MAOIs and CNS stimulants, including dexmethylphenidate hydrochloride tablets, can cause hypertensive crisis. Potential outcomes include death, stroke, myocardial infarction, aortic dissection, ophthalmological complications, eclampsia, pulmonary edema, and renal failure [see Contraindications (4)]. Intervention Concomitant use of dexmethylphenidate hydrochloride tablets with MAOIs or within 14 days after discontinuing MAOI treatment is contraindicated. Antihypertensive Drugs Clinical impact Dexmethylphenidate hydrochloride tablets may decrease the effectiveness of drugs used to treat hypertension [see Warnings and Precautions (5.3)]. Intervention Adjust the dosage of the antihypertensive drug as needed. Halogenated Anesthetics Clinical impact Concomitant use of halogenated anesthetics and dexmethylphenidate hydrochloride tablets may increase the risk of sudden blood pressure and heart rate increase during surgery. Intervention Monitor blood pressure and avoid use of dexmethylphenidate hydrochloride tablets in patients being treated with anesthetics on the day of surgery. Risperidone Clinical impact Combined use of methylphenidate with risperidone when there is a change, whether an increase or decrease, in dosage of either or both medications, may increase the risk of extrapyramidal symptoms (EPS) Intervention Monitor for signs of EPS -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ADHD medications, including dexmethylphenidate hydrochloride tablets, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for ADHD medications at 1-866-961-2388 or visiting https://womensmentalhealth.org/adhd-medications/.
Risk Summary
Dexmethylphenidate is the d-threo enantiomer of racemic methylphenidate. Published studies and postmarketing reports on methylphenidate use during pregnancy have not identified a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. There may be risks to the fetus associated with the use of CNS stimulants use during pregnancy (see Clinical Considerations). Embryo-fetal development studies in rats showed delayed fetal skeletal ossification at doses up to 5 times the maximum recommended human dose (MRHD) of 20 mg/day given to adults based on plasma levels. A decrease in pup weight in males was observed in a pre- and post-natal development study with oral administration of methylphenidate to rats throughout pregnancy and lactation at doses 5 times the MRHD of 20 mg/day given to adults based on plasma levels. Plasma levels in adults were comparatively similar to plasma levels in adolescents (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
CNS stimulants, such as dexmethylphenidate hydrochloride tablets, can cause vasoconstriction and thereby decrease placental perfusion. No fetal and/or neonatal adverse reactions have been reported with the use of therapeutic doses of methylphenidate during pregnancy; however, premature delivery and low birth weight infants have been reported in amphetamine-dependent mothers.
Data
Animal Data
In embryo-fetal development studies conducted in rats and rabbits, dexmethylphenidate was administered orally at doses of up to 20 and 100 mg/kg/day, respectively, during the period of organogenesis. No evidence of malformations was found in either the rat or rabbit study; however, delayed fetal skeletal ossification was observed at the highest dose level in rats. When dexmethylphenidate was administered to rats throughout pregnancy and lactation at doses of up to 20 mg/kg/day, post-weaning body weight gain was decreased in male offspring at the highest dose, but no other effects on postnatal development were observed. At the highest doses tested, plasma levels [area under the curves (AUCs)] of dexmethylphenidate in pregnant rats and rabbits were approximately 5 and 1 times, respectively, those in adults dosed with the MRHD of 20 mg/day.
Racemic methylphenidate has been shown to cause malformations (increased incidence of fetal spina bifida) in rabbits when given in doses of 200 mg/kg/day throughout organogenesis.
8.2 Lactation
Risk Summary
Dexmethylphenidate is the d-threo enantiomer of racemic methylphenidate. Limited published literature, based on milk sampling from seven mothers' reports that methylphenidate is present in human milk, which resulted in infant doses of 0.16% to 0.7% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 1.1 and 2.7. There are no reports of adverse effects on the breastfed infant and no effects on milk production. Long-term neurodevelopmental effects on infants from stimulant exposure are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for dexmethylphenidate hydrochloride tablets and any potential adverse effects on the breastfed infant from dexmethylphenidate hydrochloride tablets or from the underlying maternal condition.
Clinical Considerations
Monitor breastfeeding infants for adverse reactions, such as agitation, insomnia, anorexia, and reduced weight gain.
8.4 Pediatric Use
The safety and effectiveness of dexmethylphenidate hydrochloride tablets have been established in pediatric patients aged 6 to 17 years in two adequate and well-controlled clinical trials [see Clinical Studies (14)].
The safety and effectiveness of dexmethylphenidate hydrochloride tablets in pediatric patients aged less than 6 years have not been established.
The long-term efficacy of dexmethylphenidate hydrochloride tablets in pediatric patients has not been established.
Long Term Suppression of Growth
Growth should be monitored during treatment with stimulants, including dexmethylphenidate hydrochloride tablets. Pediatric patients who are not growing or gaining weight as expected may need to have their treatment interrupted [see Warnings and Precautions (5.7)].
Juvenile Animal Toxicity Data
Rats treated with racemic methylphenidate early in the postnatal period through sexual maturation demonstrated a decrease in spontaneous locomotor activity in adulthood. A deficit in acquisition of a specific learning task was observed in females only. The doses at which these findings were observed are at least 6 times the MRHD of 60 mg/day given to children on a mg/m2 basis.
In a study conducted in young rats, racemic methylphenidate was administered orally at doses of up to 100 mg/kg/day for 9 weeks, starting early in the postnatal period (postnatal Day 7) and continuing through sexual maturity (postnatal week 10). When these animals were tested as adults (postnatal Weeks 13 to 14), decreased spontaneous locomotor activity was observed in males and females previously treated with 50 mg/kg/day (approximately 4 times the MRHD of 60 mg of racemic methylphenidate given to children on a mg/m2 basis) or greater, and a deficit in the acquisition of a specific learning task was seen in females exposed to the highest dose (8 times the MRHD given to children on a mg/m2 basis). The no effect level for juvenile neurobehavioral development in rats was 5 mg/kg/day (approximately 0.5 times the MRHD given to children on a mg/m2 basis). The clinical significance of the long-term behavioral effects observed in rats is unknown.
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Dexmethylphenidate hydrochloride tablets contains dexmethylphenidate hydrochloride, a Schedule II controlled substance.
9.2 Abuse
Dexmethylphenidate hydrochloride tablets have a high potential for abuse and misuse which can lead to the development of a substance use disorder, including addiction [see Warnings and Precautions (5.1)]. Dexmethylphenidate hydrochloride can be diverted for non-medical use into illicit channels or distribution.
Abuse is the intentional non-therapeutic use of a drug, even once, to achieve a desired psychological or physiological effect. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence.
Misuse and abuse of methylphenidate may cause increased heart rate, respiratory rate, or blood pressure; sweating; dilated pupils; hyperactivity; restlessness; insomnia; decreased appetite; loss of coordination; tremors; flushed skin; vomiting; and/or abdominal pain. Anxiety, psychosis, hostility, aggression, and suicidal or homicidal ideation have also been observed with CNS stimulants abuse and/or misuse. Misuse and abuse of CNS stimulants, including dexmethylphenidate hydrochloride, can result in overdose and death [see Overdosage (10)], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
9.3 Dependence
Physical Dependence
Dexmethylphenidate hydrochloride may produce physical dependence. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug.
Withdrawal signs and symptoms after abrupt discontinuation or dose reduction following prolonged use of CNS stimulants including dexmethylphenidate hydrochloride include dysphoric mood; depression; fatigue; vivid, unpleasant dreams; insomnia or hypersomnia; increased appetite; and psychomotor retardation or agitation.
Tolerance
Dexmethylphenidate hydrochloride may produce tolerance. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose).
-
10 OVERDOSAGE
Clinical Effects of Overdose
Overdose of CNS stimulants is characterized by the following sympathomimetic effects:
- Cardiovascular effects including tachyarrhythmias, and hypertension or hypotension. Vasospasm, myocardial infarction, or aortic dissection may precipitate sudden cardiac death. Takotsubo cardiomyopathy may develop.
- CNS effects including psychomotor agitation, confusion, and hallucinations. Serotonin syndrome, seizures, cerebral vascular accidents, and coma may occur.
- Life-threatening hyperthermia (temperatures greater than 104°F) and rhabdomyolysis may develop.
Overdose Management
Consider the possibility of multiple drug ingestion. Because methylphenidate has a large volume of distribution and is rapidly metabolized, dialysis is not useful. Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
-
11 DESCRIPTION
Dexmethylphenidate hydrochloride tablets contains dexmethylphenidate hydrochloride, a CNS stimulant. Dexmethylphenidate hydrochloride is the d-threo enantiomer of racemic methylphenidate hydrochloride, which is a 50/50 mixture of the d-threo and l-threo-enantiomers. Dexmethylphenidate hydrochloride is a central nervous system (CNS) stimulant, available in 3 tablet strengths. Dexmethylphenidate hydrochloride tablet is available as 2.5 mg, 5 mg, and 10 mg strength tablets for oral administration.
Chemically, dexmethylphenidate hydrochloride is methyl α-phenyl-2-piperidineacetate hydrochloride, (R,R’)-(+)-. Its molecular formula is C14H19NO2•HCl. Its structural formula is:
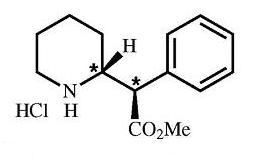
Note: * = asymmetric carbon centers
Dexmethylphenidate hydrochloride is a white to off-white powder. Its solutions are acid to litmus. It is freely soluble in water and in methanol, soluble in alcohol, and slightly soluble in chloroform and in acetone. Its molecular weight is 269.77 g/mol.
Inactive ingredients: lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, and FD&C Blue No.1 aluminum lake (2.5 mg tablets), D&C Yellow Lake No. 10 (5 mg tablets); the 10 mg tablets contain no dye.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dexmethylphenidate hydrochloride is a CNS stimulant. The mode of therapeutic action in ADHD is not known.
12.2 Pharmacodynamics
Pharmacodynamics
Dexmethylphenidate is the more pharmacologically active d-enantiomer of racemic methylphenidate. Methylphenidate blocks the reuptake of norepinephrine and dopamine into the presynaptic neuron and increase the release of these monoamines into the extraneuronal space.
Cardiac Electrophysiology
A formal QT study has not been conducted in patients taking dexmethylphenidate; however, a large QT effect is not expected. At the recommended maximum total daily dosage of 40 mg, dexmethylphenidate extended-release capsule does not prolong the QTc interval to any clinically relevant extent.
12.3 Pharmacokinetics
Absorption
Dexmethylphenidate hydrochloride is readily absorbed following oral administration of dexmethylphenidate hydrochloride tablets. In patients with ADHD, plasma dexmethylphenidate concentrations increase rapidly, reaching a maximum in the fasted state at about 1 to 1.5 hours postdose. No differences in the pharmacokinetics of dexmethylphenidate were noted following single and repeated twice daily dosing, thus indicating no significant drug accumulation in children with ADHD.
After single dose administration of dexmethylphenidate hydrochloride tablets to pediatric patients, dexmethylphenidate exposure (Cmax and AUC0-inf) showed dose-proportional increase in the range of 2.5 mg to 10 mg. Comparable plasma dexmethylphenidate levels were achieved following single dl-threo-methylphenidate HCl doses given as capsules in twice the total mg amount (equimolar with respect to dexmethylphenidate).
Approximately 90% of the dose is absorbed after oral administration of radiolabeled racemic methylphenidate. However, due to first pass metabolism the mean absolute bioavailability of dexmethylphenidate when administered in various formulations was 22% to 25%.
Effect of Food
High fat breakfast did not significantly affect Cmax or AUC0-inf of dexmethylphenidate when two 10 mg dexmethylphenidate hydrochloride tablets were administered, but delayed Tmax from 1.5 hours post dose to 2.9 hours post dose.
Distribution
The plasma protein binding of dexmethylphenidate is not known; racemic methylphenidate is bound to plasma proteins by 12% to 15%, independent of concentration. Dexmethylphenidate shows a volume of distribution of 2.65 ± 1.11 L/kg.
Elimination
Plasma dexmethylphenidate concentrations declined exponentially following oral administration of dexmethylphenidate hydrochloride tablets. Intravenous dexmethylphenidate was eliminated with a mean clearance of 0.40 ± 0.12 L/hr/kg. The mean terminal elimination half-life of dexmethylphenidate was approximately 2.2 hours.
Metabolism
In humans, dexmethylphenidate is metabolized primarily via de-esterification to d-α-phenyl-piperidine acetic acid (also known as d-ritalinic acid). This metabolite has little or no pharmacological activity. There is little or no in vivo interconversion to the l-threo-enantiomer.
Excretion
After oral dosing of radiolabeled racemic methylphenidate in humans, about 90% of the radioactivity was recovered in urine. The main urinary metabolite of racemic dl-methylphenidate was dl-ritalinic acid, accountable for approximately 80% of the dose. Urinary excretion of parent compound accounted for 0.5% of an intravenous dose.
Studies in Special Populations
Male and Female Patients
Pharmacokinetic parameters were similar for boys and girls (mean age 10 years).
In a single dose study conducted in adults, the mean dexmethylphenidate AUC0-inf values (adjusted for body weight) following single two 10 mg doses of dexmethylphenidate hydrochloride were 25% to 35% higher in adult female volunteers (n = 6) compared to male volunteers (n = 9). Both Tmax and t1/2 were comparable for males and females.
Racial or Ethnic Groups
There is insufficient experience with the use of dexmethylphenidate hydrochloride to detect ethnic variations in pharmacokinetics.
Pediatric Patients
The pharmacokinetics of dexmethylphenidate after dexmethylphenidate hydrochloride tablets administration have not been studied in children less than 6 years of age. When single doses of dexmethylphenidate hydrochloride tablets were given to children between the ages of 6 to 12 years and healthy adult volunteers, Cmax of dexmethylphenidate was similar, however, pediatric patients showed somewhat lower AUCs compared to the adults.
Patients with Renal Impairment
There is no experience with the use of dexmethylphenidate hydrochloride tablets in patients with renal impairment. Since renal clearance is not an important route of methylphenidate clearance, renal impairment is expected to have little effect on the pharmacokinetics of dexmethylphenidate hydrochloride tablets.
Patients with Hepatic Impairment
There is no experience with the use of dexmethylphenidate hydrochloride tablets in patients with hepatic impairment.
Drug Interaction Studies
Methylphenidate is not metabolized by cytochrome P450 (CYP) isoenzymes to a clinically relevant extent. Inducers or inhibitors of CYPs are not expected to have any relevant impact on methylphenidate pharmacokinetics. Conversely, the d- and l-enantiomers of methylphenidate did not relevantly inhibit CYP1A2, 2C8, 2C9, 2C19, 2D6, 2E1, or 3A. Clinically, methylphenidate coadministration did not increase plasma concentrations of the CYP2D6 substrate desipramine.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenesis
Lifetime carcinogenicity studies have not been carried out with dexmethylphenidate. In a lifetime carcinogenicity study carried out in B6C3F1 mice, racemic methylphenidate caused an increase in hepatocellular adenomas, and in males only, an increase in hepatoblastomas was seen at a daily dose of approximately 60 mg/kg/day. This dose is approximately 2 times the MRHD of 60 mg/day of racemic methylphenidate given to children on a mg/m2 basis. Hepatoblastoma is a relatively rare rodent malignant tumor type. There was no increase in total malignant hepatic tumors. The mouse strain used is sensitive to the development of hepatic tumors and the significance of these results to humans is unknown.
Racemic methylphenidate did not cause any increase in tumors in a lifetime carcinogenicity study carried out in F344 rats; the highest dose used was approximately 45 mg/kg/day, which is approximately 4 times the MRHD (children) of 60 mg/day of racemic methylphenidate on a mg/m2 basis.
In a 24-week carcinogenicity study with racemic methylphenidate in the transgenic mouse strain p53+/-, which is sensitive to genotoxic carcinogens, there was no evidence of carcinogenicity. Male and female mice were fed diets containing the same concentrations as in the lifetime carcinogenicity study; the high-dose group was exposed to 60-74 mg/kg/day of racemic methylphenidate.
Mutagenesis
Dexmethylphenidate was not mutagenic in the in vitro Ames reverse mutation assay, in the in vitro mouse lymphoma cell forward mutation assay, or in the in vivo mouse bone marrow micronucleus test. In an in vitro assay using cultured Chinese Hamster Ovary cells treated with racemic methylphenidate, sister chromatid exchanges and chromosome aberrations were increased, indicative of a weak clastogenic response.
Impairment of Fertility
No human data on the effect of methylphenidate on fertility are available.
Fertility studies have not been conducted with dexmethylphenidate. Racemic methylphenidate did not impair fertility in male or female mice that were fed diets containing the drug in an 18-week continuous breeding study. The study was conducted at doses of up to 160 mg/kg/day, approximately 10 times the MRHD of 60 mg/day of racemic methylphenidate given adolescents on a mg/m2 basis.
-
14 CLINICAL STUDIES
The efficacy of dexmethylphenidate hydrochloride tablets for the treatment of ADHD was established in two double-blind, parallel-group, placebo-controlled trials in untreated or previously treated patients (ages 6 to 17 years old) who met The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for ADHD inattentive, hyperactive-impulsive, or combined inattentive/hyperactive-impulsive subtypes. The sample was predominantly younger (ages 6 to 12 years); thus, the findings are most pertinent to this age group.
In Study 1, patients were randomized to receive either dexmethylphenidate hydrochloride tablets (5, 10, or 20 mg/day total dose), racemic methylphenidate HCl (10, 20, or 40 mg/day total dose), or placebo in a multicenter, 4-week, parallel group study in 132 pediatric patients. Patients received study medication twice daily separated by a 3.5 to 5.5 hours interval. Treatment was initiated with the lowest dose, and doses could be doubled at weekly intervals, depending on clinical response and tolerability, up to the maximum dose. The primary outcome was change from baseline to week 4 of the average score (an average of 2 ratings during the week) of the teacher’s version of the Swanson, Nolan and Pelham (SNAP)-ADHD Rating Scale. This 18 item scale measures ADHD symptoms of inattention and hyperactivity/impulsivity, rated on a scale of 0 (Not at All) to 3 (Very Much). Patients treated with dexmethylphenidate hydrochloride tablets showed a statistically significant improvement in symptom scores from baseline over patients who received placebo (Table 3).
Table 3: Summary of Efficacy Results From ADHD Acute-Phase Study in Pediatric Patients (6 - 17 years) (Study 1) Abbreviations: ADHD, attention deficit hyperactivity disorder; SD, standard deviation; SNAP; swanson, Nolan and Pelham; n, number of patients available at the assessment time point.
a Average of two ratings.
b Statistically significantly different from placebo.Study number Treatment group Primary efficacy measure: teacher SNAP-ADHD total Scorea Mean baseline score (SD) Mean change from baseline Week 4 score (SD) Study 1 Dexmethylphenidate hydrochloride tablets 5-20 mg/dayb
(n = 44)1.4 (0.7) (n = 42) - 0.7 (0.7) (n = 42) Placebo (n = 42) 1.6 (0.7) (n = 41) - 0.2 (0.7) (n = 39) Study 2 was a multicenter, placebo-controlled, double-blind, 2-week treatment withdrawal study in 75 children (ages 6 to 12 years) who were responders during a 6-week, open-label initial treatment period. Children took study medication twice a day separated by a 3.5 to 5.5 hour interval. The primary outcome was proportion of treatment failures at the end of the 2-week withdrawal phase, where treatment failure was defined as a rating of 6 (much worse) or 7 (very much worse) on the Investigator Clinical Global Impression - Improvement (CGI-I). Patients continued on dexmethylphenidate hydrochloride tablets showed a statistically significant lower rate of failure over patients who received placebo (Table 4).
Table 4: Summary of Efficacy Results From ADHD Randomized Withdrawal Study in Pediatric Patients (6 - 17 years) (Study 2) Abbreviation: ADHD, attention deficit hyperactivity disorder.
a One patient did not have the value at Visit 10 and hence not included in this analysis.
b Statistically significantly different from placebo.Study number Treatment group Primary efficacy measure: proportion of treatment failurea Number of treatment failures / Number of randomized patients Percentage Study 2 Dexmethylphenidate hydrochloride tablets 5-20 mg/dayb 6/35 17.1% Placebo 25/40 62.5% -
16 HOW SUPPLIED/STORAGE AND HANDLING
Dexmethylphenidate Hydrochloride Tablets, 2.5 mg are blue, round-shaped, convex tablets debossed with 91 on one side and plain on the other side, supplied in bottles of 100 NDC 27808-091-01
Dexmethylphenidate Hydrochloride Tablets, 5 mg are yellow, round-shaped, convex tablets debossed with 92 on one side and plain on the other side, supplied in bottles of 100 NDC 27808-092-01
Dexmethylphenidate Hydrochloride Tablets, 10 mg are white to off white, round-shaped, convex tablets debossed with 93 on one side and plain on the other side, supplied in bottles of 100 NDC 27808-093-01
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
Dispense in tight, light-resistant container as defined in the USP, with a child-resistant closure.
Protect from light and moisture.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Abuse, Misuse, and Addiction
Educate patients and their families about the risks of abuse, misuse, and addiction of dexmethylphenidate hydrochloride, which can lead to overdose and death, and proper disposal of any unused drug [see Warnings and Precautions (5.1), Drug Abuse and Dependence (9.2), Overdosage (10)]. Advise patients to store dexmethylphenidate hydrochloride tablets in a safe place, preferably locked, and instruct patients to not give dexmethylphenidate hydrochloride tablets to anyone else.
Risks to Patients with Serious Cardiac Disease
Advise patients that there are potential risks to patients with serious cardiac disease, including sudden death, with dexmethylphenidate hydrochloride tablets use. Instruct patients to contact a healthcare provider immediately if they develop symptoms, such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease [see Warnings and Precautions (5.2)].
Increased Blood Pressure and Heart Rate
Instruct patients that dexmethylphenidate hydrochloride tablets can cause elevations of their blood pressure and pulse rate [see Warnings and Precautions (5.3)].
Psychiatric Adverse Reactions
Advise patients that dexmethylphenidate hydrochloride tablets, at recommended doses, can cause psychotic or manic symptoms, even in patients without prior history of psychotic symptoms or mania [see Warnings and Precautions (5.4)].
Priapism
Advise patients of the possibility of painful or prolonged penile erections (priapism). Instruct them to seek immediate medical attention in the event of priapism [see Warnings and Precautions (5.5)].
Circulation Problems in Fingers and Toes [Peripheral Vasculopathy, Including Raynaud’s Phenomenon]
Instruct patients beginning treatment with dexmethylphenidate hydrochloride tablets about the risk of peripheral vasculopathy, including Raynaud’s phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red. Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking dexmethylphenidate hydrochloride tablets. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients [see Warnings and Precautions (5.6)].
Long-Term Suppression of Growth in Pediatric Patients
Advise patients that dexmethylphenidate hydrochloride tablets may cause slowing of growth and weight loss [see Warnings and Precautions (5.7)].
Increased Intraocular Pressure (IOP) and Glaucoma
Advise patients that IOP and glaucoma may occur during treatment with dexmethylphenidate hydrochloride [see Warnings and Precautions (5.9)].
Motor and Verbal Tics, and Worsening of Tourette’s Syndrome
Advise patients that motor and verbal tics and worsening of Tourette’s Syndrome may occur during treatment with dexmethylphenidate hydrochloride. Instruct patients to notify their healthcare provider if emergence of new tics or worsening of tics or Tourette’s syndrome occurs [see Warnings and Precautions (5.10)].
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in patients exposed to ADHD medications, including dexmethylphenidate hydrochloride tablets, during pregnancy [see Use in Specific Populations (8.1)].
Manufactured by:
Tris Pharma, Inc.
Monmouth Junction, NJ 08852LB8408
Rev. 05
11/2023 -
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration
Revised:11/2023
MEDICATION GUIDE
Dexmethylphenidate Hydrochloride Tablets CII
(dex-meth-ill-FEN-ih-date)What is the most important information I should know about Dexmethylphenidate hydrochloride tablets?
Dexmethylphenidate hydrochloride tablets may cause serious side effects, including:- Abuse, misuse, and addiction. Dexmethylphenidate hydrochloride tablets have a high chance for abuse and misuse and may lead to substance use problems, including addiction. Misuse and abuse of dexmethylphenidate hydrochloride tablets, other methylphenidate containing medicines, and amphetamine containing medicines, can lead to overdose and death. The risk of overdose and death is increased with higher doses of dexmethylphenidate hydrochloride tablets or when it is used in ways that are not approved, such as snorting or injection.
- Your healthcare provider should check you or your child’s risk for abuse, misuse, and addiction before starting treatment with dexmethylphenidate hydrochloride tablets and will monitor you or your child during treatment.
- Dexmethylphenidate hydrochloride tablets may lead to physical dependence after prolonged use, even if taken as directed by your healthcare provider.
- Do not give dexmethylphenidate hydrochloride tablets to anyone else. See “What is Dexmethylphenidate hydrochloride tablets?” for more information.
- Keep dexmethylphenidate hydrochloride tablets in a safe place and properly dispose of any unused medicine. See “How should I store dexmethylphenidate hydrochloride tablets?” for more information.
- Tell your healthcare provider if you or your child have ever abused or been dependent on alcohol, prescription medicines, or street drugs.
-
Risks for people with serious heart disease. Sudden death has happened in people who have heart defects or other serious heart disease.
Your healthcare provider should check you or your child carefully for heart problems before starting dexmethylphenidate hydrochloride tablets. Tell your healthcare provider if you or your child have any heart problems, heart disease, or heart defects.
Call your healthcare provider or go to the nearest hospital emergency room right away if you or your child has any signs of heart problems, such as chest pain, shortness of breath, or fainting while taking dexmethylphenidate hydrochloride tablets.
- Increased blood pressure and heart rate. Your healthcare provider should check you or your child’s blood pressure and heart rate regularly during treatment with dexmethylphenidate hydrochloride tablets.
-
Mental (psychiatric) problems:
All Patients
- new or worse behavior and thought problems
- new or worse bipolar illness
- new psychotic symptoms (such as hearing voices, believing things that are not true, are suspicious) or new manic symptoms
Tell your healthcare provider about any mental problems you or your child have, or about a family history of suicide, bipolar illness, or depression.
Call your healthcare provider right away if you or your child have any new or worsening mental symptoms or problems while taking dexmethylphenidate hydrochloride tablets, especially seeing or hearing things that are not real, believing things that are not real, or are suspicious.What is Dexmethylphenidate hydrochloride tablets?
- Dexmethylphenidate hydrochloride tablets are a central nervous system stimulant (CNS) prescription medicine. It is used for the treatment of Attention-Deficit Hyperactivity Disorder (ADHD). Dexmethylphenidate hydrochloride tablets may help increase attention and decrease impulsiveness and hyperactivity in patients with ADHD.
- Dexmethylphenidate hydrochloride tablets should be used as a part of a total treatment program for ADHD that may include counseling or other therapies.
Dexmethylphenidate hydrochloride tablets is a federally controlled substance (CII) because it contains dexmethylphenidate that can be a target for people who abuse prescription medicines or street drugs. Keep dexmethylphenidate hydrochloride tablets in a safe place to protect it from theft. Never give your dexmethylphenidate hydrochloride tablets to anyone else because it may cause death or harm them. Selling or giving away dexmethylphenidate hydrochloride tablets may harm others and is against the law.
Who should not take Dexmethylphenidate hydrochloride tablets:
Dexmethylphenidate hydrochloride tablets should not be taken if you or your child:- are allergic to methylphenidate hydrochloride, or any of the ingredients in dexmethylphenidate hydrochloride tablets. See the end of this Medication Guide for a complete list of ingredients in dexmethylphenidate hydrochloride tablets.
- are taking or have taken within the past 14 days an anti-depression medicine called a monoamine oxidase inhibitor (MAOI).
Dexmethylphenidate hydrochloride tablets may not be right for you or your child. Before starting dexmethylphenidate hydrochloride tablets, tell your or your child’s healthcare provider about all health conditions (or a family history of), including:
- heart problems, heart disease, heart defects, or high blood pressure
- mental problems, including psychosis, mania, bipolar illness, or depression
- circulation problems in fingers or toes
- have eye problems, including increased pressure in your eye, glaucoma, or problems with your close-up vision (farsightedness)
- have or had repeated movements or sounds (tics) or Tourette’s syndrome, or have a family history of tics or Tourette’s syndrome.
- if you are pregnant or plan to become pregnant. It is not known if dexmethylphenidate hydrochloride tablets will harm your unborn baby.
- There is a pregnancy registry for females who are exposed to ADHD medications, including dexmethylphenidate hydrochloride tablets during pregnancy. The purpose of the registry is to collect information about the health of females exposed to dexmethylphenidate hydrochloride tablets and their baby. If you or your child becomes pregnant during treatment with dexmethylphenidate hydrochloride tablets, talk to your healthcare provider about registering with the National Pregnancy Registry of ADHD medications at 1-866-961-2388 or visit online at https://womensmentalhealth.org/adhd-medications/.
- if you are breastfeeding or plan to breastfeed. Dexmethylphenidate hydrochloride passes into your breast milk. Talk to your healthcare provider about the best way to feed the baby during treatment with dexmethylphenidate hydrochloride tablets.
Tell your healthcare provider about all of the medicines that you or your child takes, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Dexmethylphenidate hydrochloride tablets and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be adjusted while taking dexmethylphenidate hydrochloride tablets.
Your healthcare provider will decide whether dexmethylphenidate hydrochloride tablets can be taken with other medicines.
Especially tell your healthcare provider if you or your child takes:
- blood pressure medicines (anti-hypertensive)
Know the medicines that you or your child takes. Keep a list of your medicines with you to show your healthcare provider and pharmacist.
- You should not take dexmethylphenidate hydrochloride tablets on the day of your operation if a certain type of anesthetic is used. This is because there is a chance of a sudden rise in blood pressure and heart rate during the operation.
Do not start any new medicine while taking dexmethylphenidate hydrochloride tablets without talking to your healthcare provider first.
How should Dexmethylphenidate hydrochloride tablets be taken?
- Take dexmethylphenidate hydrochloride tablets exactly as prescribed. Your healthcare provider may adjust the dose until it is right for you or your child.
- Take dexmethylphenidate hydrochloride tablets twice daily, at least 4 hours apart.
- Dexmethylphenidate hydrochloride tablets may be taken with or without food.
- Your healthcare provider may do regular checks of the blood, heart, and blood pressure while taking dexmethylphenidate hydrochloride tablets.
- Children should have their height and weight checked often while taking dexmethylphenidate hydrochloride tablets. Dexmethylphenidate hydrochloride tablets treatment may be stopped if a problem is found during these check-ups.
If you or your child take too much dexmethylphenidate hydrochloride tablets, call your healthcare provider or Poison Help line at 1-800-222-1222 or go to the nearest hospital emergency room right away.
What are the possible side effects of Dexmethylphenidate hydrochloride tablets?
Dexmethylphenidate hydrochloride tablets may cause serious side effects, including:- see “What is the most important information I should know about dexmethylphenidate hydrochloride tablets?” for information on reported heart and mental problems.
- painful and prolonged erections (priapism) have occurred with methylphenidate. If you or your child develops priapism, seek medical help right away. Because of the potential for lasting damage, priapism should be evaluated by a healthcare provider immediately.
-
circulation problems in fingers and toes (peripheral vasculopathy, including Raynaud’s phenomenon):
o fingers or toes may feel numb, cool, painful
o fingers or toes may change color from pale, to blue, to red
Tell your healthcare provider if you or your child have, numbness, pain, skin color change, or sensitivity to temperature in the fingers or toes.
- Call your healthcare provider right away if you have or your child has any signs of unexplained wounds appearing on fingers or toes while taking dexmethylphenidate hydrochloride tablets.
- Slowing of growth (height and weight) in children. Children should have their height and weight checked often during treatment with dexmethylphenidate hydrochloride tablets. Dexmethylphenidate hydrochloride tablets treatment may be stopped if your child is not growing or gaining weight.
- Eye problems (increased pressure in the eye and glaucoma). Call your healthcare provider right away if you or your child develop changes in your vision or eye pain, swelling, or redness.
- New or worsening tics or worsening Tourette’s syndrome. Tell your healthcare provider if you or your child get any new or worsening tics or worsening Tourette’s syndrome during treatment with dexmethylphenidate hydrochloride tablets.
- Common side effects include:
• abdominal pain • fever • anorexia • nausea Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store Dexmethylphenidate hydrochloride tablets?
- Store dexmethylphenidate hydrochloride tablets in a safe place and in a tightly closed container at room temperature between 68°F to 77°F (20°C to 25°C).
- Protect from light.
- Dispose of remaining, unused, or expired dexmethylphenidate hydrochloride tablets by a medicine take-back program at a U.S. Drug Enforcement Administration (DEA) authorized collection site. If no take-back program or DEA authorized collector is available, mix dexmethylphenidate hydrochloride tablets with an undesirable, nontoxic substance, such as dirt, cat litter, or used coffee grounds to make it less appealing to children and pets. Place the mixture in a container, such as a sealed plastic bag and throw away (discard) dexmethylphenidate hydrochloride tablets in the household trash. Visit www.fda.gov/drugdisposal for additional information on disposal of unused medicines.
- Keep dexmethylphenidate hydrochloride tablets and all medicines out of the reach of children.
General information about the safe and effective use of Dexmethylphenidate hydrochloride tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about dexmethylphenidate hydrochloride tablets that is written for healthcare professionals. Do not use dexmethylphenidate hydrochloride tablets for a condition for which it was not prescribed. Do not give dexmethylphenidate hydrochloride tablets to other people, even if they have the same symptoms that you have. It may harm them and it is against the law.
What are the ingredients in Dexmethylphenidate hydrochloride tablets?
Active ingredient: dexmethylphenidate hydrochloride
Inactive ingredients: lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, and FD&C Blue No.1 aluminum lake (2.5 mg tablets), D&C Yellow Lake No. 10 (5 mg tablets); the 10 mg tablet contains no dye.
Manufactured by:
Tris Pharma, Inc.
Monmouth Junction, NJ 08852LB8409
Rev. 05 - PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DEXMETHYLPHENIDATE HYDROCHLORIDE
dexmethylphenidate hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:27808-091 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXMETHYLPHENIDATE HYDROCHLORIDE (UNII: 1678OK0E08) (DEXMETHYLPHENIDATE - UNII:M32RH9MFGP) DEXMETHYLPHENIDATE HYDROCHLORIDE 2.5 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color blue (Blue) Score no score Shape ROUND (91) Size 7mm Flavor Imprint Code 91 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:27808-091-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/30/2001 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207901 11/30/2001 DEXMETHYLPHENIDATE HYDROCHLORIDE
dexmethylphenidate hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:27808-092 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXMETHYLPHENIDATE HYDROCHLORIDE (UNII: 1678OK0E08) (DEXMETHYLPHENIDATE - UNII:M32RH9MFGP) DEXMETHYLPHENIDATE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color yellow Score no score Shape ROUND Size 7mm Flavor Imprint Code 92 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:27808-092-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 11/30/2001 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207901 11/30/2001 DEXMETHYLPHENIDATE HYDROCHLORIDE
dexmethylphenidate hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:27808-093 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXMETHYLPHENIDATE HYDROCHLORIDE (UNII: 1678OK0E08) (DEXMETHYLPHENIDATE - UNII:M32RH9MFGP) DEXMETHYLPHENIDATE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color white Score no score Shape ROUND Size 7mm Flavor Imprint Code 93 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:27808-093-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 11/30/2001 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207901 11/30/2001 Labeler - Tris Pharma Inc (947472119)






