Label: PHENOBARBITAL tablet
-
NDC Code(s):
16571-669-01,
16571-669-10,
16571-669-50,
16571-670-01, view more16571-670-10, 16571-670-50, 16571-672-01, 16571-672-10, 16571-672-50, 16571-674-01, 16571-674-10, 16571-674-50
- Packager: Rising Pharma Holdings, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated July 1, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Phenobarbital is a barbituric acid derivative for oral administration and occurs as a white, odorless, slightly bitter powder that is soluble in chloroform, freely soluble in alcohol or ether, and slightly soluble in water. Its saturated solution has a pH of about 5.6. Chemically, it is 5-ethyl-5- phenylbarbituric acid with the molecular formula C 12 H 12 N2O3 (232.24). The structural formula is as follows:
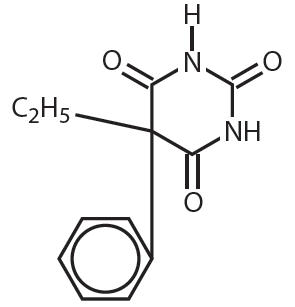
Each Phenobarbital Tablet, USP contains 15 mg, 30 mg, 60 mg or 100 mg of phenobarbital, USP.
Inactive ingredients include: colloidal silicon dioxide, lactose monohydrate, microcrystalline cellulose, sodium starch glycolate and magnesium stearate. - CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Phenobarbital is contraindicated in patients who are hypersensitive to barbiturates. In such patients, severe hepatic damage can occur from ordinary doses and is usually associated with dermatitis and involvement of parenchymatous organs. A personal or familial history of acute intermittent porphyria represents one of the few absolute contraindications to the use of barbiturates. Phenobarbital is also contraindicated in patients with marked impairment of liver function, or respiratory disease in which dyspnea or obstruction is evident. It should not be administered to persons with known previous addiction to the sedative/hypnotic group, since ordinary doses may be ineffectual and may contribute to further addiction.
- WARNINGS
-
PRECAUTIONS
General Precautions:
Barbiturates induce liver microsomal enzyme activity. This accelerates the biotransformation of various drugs and is probably part of the mechanism of the tolerance encountered with barbiturates. Phenobarbital, therefore, should be used with caution in patients with decreased liver function. This drug should also be administered cautiously to patients with a history of drug dependence or abuse (see DRUG ABUSE AND DEPENDENCE).
Phenobarbital may decrease the potency of coumarin anticoagulants; therefore, patients receiving such concomitant therapy should have more frequent prothrombin determinations. As with other sedatives and hypnotics, elderly or debilitated patients may react to barbiturates with marked excitement, depression, or confusion.The systemic effects of exogenous hydrocortisone and endogenous hydrocortisone (cortisol) may be diminished by phenobarbital. Thus, this product should be administered with caution to patients with borderline hypoadrenal function, regardless of whether it is of pituitary or of primary adrenal origin.
Information for Patients:
Phenobarbital may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery. The patient should be cautioned accordingly.
Drug Interactions:
Phenobarbital in combination with alcohol, tranquilizers, and other central nervous system depressants has additive depressant effects, and the patient should be so advised. Patients taking this drug should be warned not to exceed the dosage recommended by their physician. Toxic effects and fatalities have occurred following overdoses of phenobarbital alone and in combination with other central nervous system depressants. Caution should be exercised in prescribing unnecessarily large amounts of phenobarbital for patients who have a history of emotional disturbances or suicidal ideation or who have misused alcohol and other CNS drugs (see OVERDOSAGE).
Usage in Pregnancy:
Pregnancy Category B - Reproduction studies have been performed in animals and have revealed no evidence of impaired fertility or harm to the fetus due to phenobarbital. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
-
ADVERSE REACTIONS
The following adverse reactions have been reported:
CNS Depression:
Residual sedation or “hangover”, drowsiness, lethargy, and vertigo. Emotional disturbances and phobias may be accentuated. In some persons, barbiturates such as phenobarbital repeatedly produce excitement rather than depression, and the patient may appear to be inebriated. Like other non-analgesic hypnotic drugs, barbiturates, such as phenobarbital, when given in the presence of pain, may cause restlessness, excitement, and even delirium. Rarely, the use of barbiturates results in localized or diffused myalgic, neuralgic, or arthritic pain, especially in psychoneurotic patients with insomnia. The pain may appear in paroxysms, is most intense in the early morning hours, and is most frequently located in the region of the neck, shoulder girdle, and upper limbs. Symptoms may last for days after the drug is discontinued.Respiratory/Circulatory:
Respiratory depression, apnea, circulatory collapse.
Allergic:
Acquired hypersensitivity to barbiturates consists chiefly in allergic reactions that occur especially in persons who tend to have asthma, urticaria, angioedema, and similar conditions. Hypersensitivity reactions in this category include localized swelling, particularly of the eyelids, cheeks, or lips, and erythematous dermatitis. Rarely, exfoliative dermatitis (e.g., Stevens-Johnson syndrome and toxic epidermal necrolysis) may be caused by phenobarbital and can prove fatal. The skin eruption may be associated with fever, delirium, and marked degenerative changes in the liver and other parenchymatous organs. In a few cases, megaloblastic anemia has been associated with the chronic use of phenobarbital.Other:
Nausea and vomiting; headache.To report SUSPECTED ADVERSE REACTIONS, contact Rising Pharma Holdings, Inc. at 1-844-874-7464, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
DRUG ABUSE AND DEPENDENCE
Controlled Substance - Phenobarbital is a Schedule IV drug.
Dependence:
Prolonged, uninterrupted use of barbiturates (particularly the short-acting drugs), even in therapeutic doses, may result in psychic and physical dependence. Withdrawal symptoms due to physical dependence following chronic use of large doses of barbiturates may include delirium, convulsions, and death. -
OVERDOSAGE
The signs and symptoms of barbiturate poisoning are referable especially to the central nervous system and the cardiovascular system. Moderate intoxication resembles alcoholic inebriation. In severe intoxication, the patient is comatose, the level of reflex activity conforming in a general way to the intensity of the central depression. The deep reflexes may persist for some time despite coexistent coma. The Babinski sign is often positive. The EEG may be of the “burst-suppression” type, with brief periods of electrical silence. The pupils may be constricted and react to light, but late in the course of barbiturate poisoning they may show hypoxic paralytic dilatation. Respiration is affected early. Breathing may be either slow or rapid and shallow; Cheyne-Stokes rhythm may be present. Respiratory minute volume is diminished, and hypoxia and respiratory acidosis may develop. The blood pressure falls, owing partly to depression of medullary vasomotor centers; partly to a direct action of the drug on the myocardium, sympathetic ganglia, and vascular smooth muscle; partly to hypoxia.
The patient thus develops a typical shock syndrome, with a weak and rapid pulse, cold and clammy skin, and a rise in the hematocrit. Respiratory complications (atelectasis, pulmonary edema, and bronchopneumonia) and renal failure are much dreaded and not infrequent concomitant of severe barbiturate poisoning. There is usually hypothermia, sometimes with temperatures as low as 32°C.
Treatment:
General management should consist of symptomatic and supportive therapy, including gastric lavage, administration of intravenous fluids, and maintenance of blood pressure, body temperature and adequate respiratory exchange. Dialysis will increase the rate of removal of barbiturates from the body fluids. Antibiotics may be required to control pulmonary complications. - DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Phenobarbital Tablets, USP 15 mg: White to off-white round, biconvex tablets debossed “T35” on one side and plain on the other side.
Available in bottles of 100 tablets, NDC 16571-670-01; 500 tablets, NDC 16571-670-50; and 1000 tablets, NDC 16571-670-10.
Phenobarbital Tablets, USP 30 mg: White to off-white round, biconvex tablets debossed “T” above and “36” below the bisect on one side and plain on the other side.
Available in bottles of 100 tablets, NDC 16571-672-01; 500 tablets, NDC 16571-672-50; and 1000 tablets, NDC 16571-672-10.
Phenobarbital Tablets, USP 60 mg: White to off-white round, biconvex tablets debossed “T” above and “37” below the bisect on one side and plain on the other side.
Available in bottles of 100 tablets, NDC 16571-674-01; 500 tablets, NDC 16571-674-50; and 1000 tablets, NDC 16571-674-10.
Phenobarbital Tablets, USP 100 mg: White to off-white round, biconvex tablets debossed “T” above and “38” below the bisect on one side and plain on the other side.
Available in bottles of 100 tablets, NDC 16571-669-01; 500 tablets, NDC 16571-669-50; and 1000 tablets, NDC 16571-669-10.Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Protect from light and moisture.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Distributed by:
Rising Pharma Holdings, Inc.
East Brunswick, NJ 08816Manufactured by:
Quagen Pharmaceuticals LLC
West Caldwell, NJ 0700652024
Rev. 07/20 -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Rising® NDC 16571-670-01
Phenobarbital*
Tablets, USP CIV
15 mg
*WARNING: May be habit forming.
100 Tablets Rx Only

Rising® NDC 16571-670-50
Phenobarbital*
Tablets, USP CIV
15 mg
*WARNING: May be habit forming.
500 Tablets Rx Only

Rising® NDC 16571-670-10
Phenobarbital*
Tablets, USP CIV
15 mg
*WARNING: May be habit forming.
1000 Tablets Rx Only

Rising® NDC 16571-672-01
Phenobarbital*
Tablets, USP CIV
30 mg
*WARNING: May be habit forming.
100 Tablets Rx Only

Rising® NDC 16571-672-50
Phenobarbital*
Tablets, USP CIV
30 mg
*WARNING: May be habit forming.
500 Tablets Rx Only

Rising® NDC 16571-672-10
Phenobarbital*
Tablets, USP CIV
30 mg
*WARNING: May be habit forming.
1000 Tablets Rx Only

Rising® NDC 16571-674-01
Phenobarbital*
Tablets, USP CIV
60 mg
*WARNING: May be habit forming.
100 Tablets Rx Only

Rising® NDC 16571-674-50
Phenobarbital*
Tablets, USP CIV
60 mg
*WARNING: May be habit forming.
500 Tablets Rx Only

Rising® NDC 16571-674-10
Phenobarbital*
Tablets, USP CIV
60 mg
*WARNING: May be habit forming.
1000 Tablets Rx Only

Rising® NDC 16571-669-01
Phenobarbital*
Tablets, USP CIV
100 mg
*WARNING: May be habit forming.
100 Tablets Rx Only

Rising® NDC 16571-669-50
Phenobarbital*
Tablets, USP CIV
100 mg
*WARNING: May be habit forming.
500 Tablets Rx Only

Rising® NDC 16571-669-10
Phenobarbital*
Tablets, USP CIV
100 mg
*WARNING: May be habit forming.
1000 Tablets Rx Only

-
INGREDIENTS AND APPEARANCE
PHENOBARBITAL
phenobarbital tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16571-670 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENOBARBITAL (UNII: YQE403BP4D) (PHENOBARBITAL - UNII:YQE403BP4D) PHENOBARBITAL 15 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (White to off-white) Score no score Shape ROUND (Round, Biconvex) Size 6mm Flavor Imprint Code T;35 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16571-670-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2020 2 NDC:16571-670-50 500 in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2020 3 NDC:16571-670-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 11/11/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/01/2020 PHENOBARBITAL
phenobarbital tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16571-672 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENOBARBITAL (UNII: YQE403BP4D) (PHENOBARBITAL - UNII:YQE403BP4D) PHENOBARBITAL 30 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (White to off-white) Score 2 pieces Shape ROUND (Round, Biconvex) Size 8mm Flavor Imprint Code T;36 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16571-672-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2020 2 NDC:16571-672-50 500 in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2020 3 NDC:16571-672-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/01/2020 PHENOBARBITAL
phenobarbital tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16571-674 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENOBARBITAL (UNII: YQE403BP4D) (PHENOBARBITAL - UNII:YQE403BP4D) PHENOBARBITAL 60 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (White to off-white) Score 2 pieces Shape ROUND (Round, Biconvex) Size 10mm Flavor Imprint Code T;37 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16571-674-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2020 2 NDC:16571-674-50 500 in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2020 3 NDC:16571-674-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/01/2020 PHENOBARBITAL
phenobarbital tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16571-669 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENOBARBITAL (UNII: YQE403BP4D) (PHENOBARBITAL - UNII:YQE403BP4D) PHENOBARBITAL 100 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (White to off-white) Score 2 pieces Shape ROUND (Round, Biconvex) Size 12mm Flavor Imprint Code T;38 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16571-669-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2020 2 NDC:16571-669-50 500 in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2020 3 NDC:16571-669-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 11/01/2020 Labeler - Rising Pharma Holdings, Inc. (835513529) Registrant - QUAGEN PHARMACEUTICALS LLC (080281331) Establishment Name Address ID/FEI Business Operations QUAGEN PHARMACEUTICALS LLC 080281331 MANUFACTURE(16571-669, 16571-670, 16571-672, 16571-674) , PACK(16571-669, 16571-670, 16571-672, 16571-674)












