Label: NO7 TIME RESISTING DAY SUNSCREEN SPF 12- octinoxate and avobenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 11489-062-01 - Packager: BCM Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 6, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
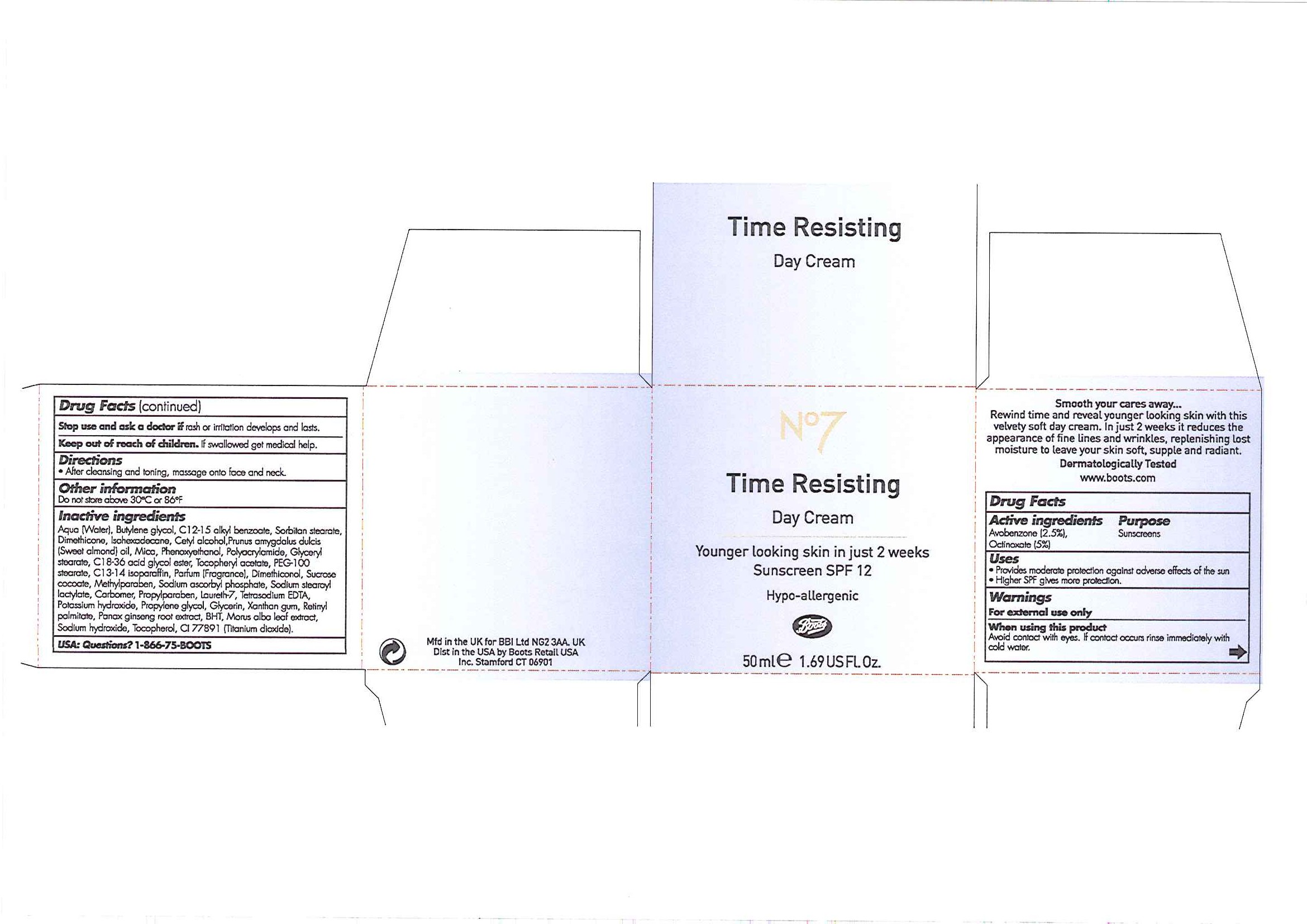
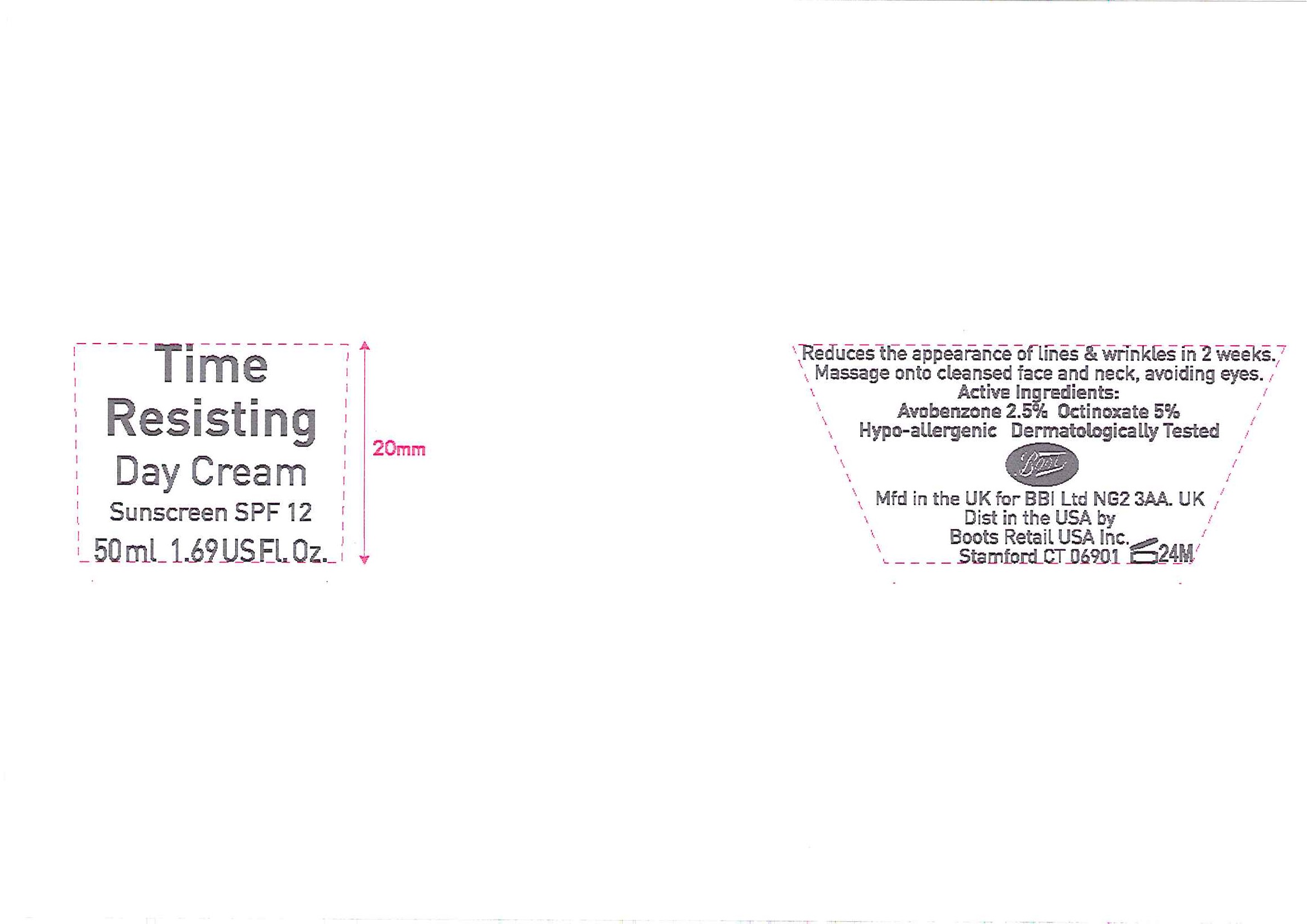
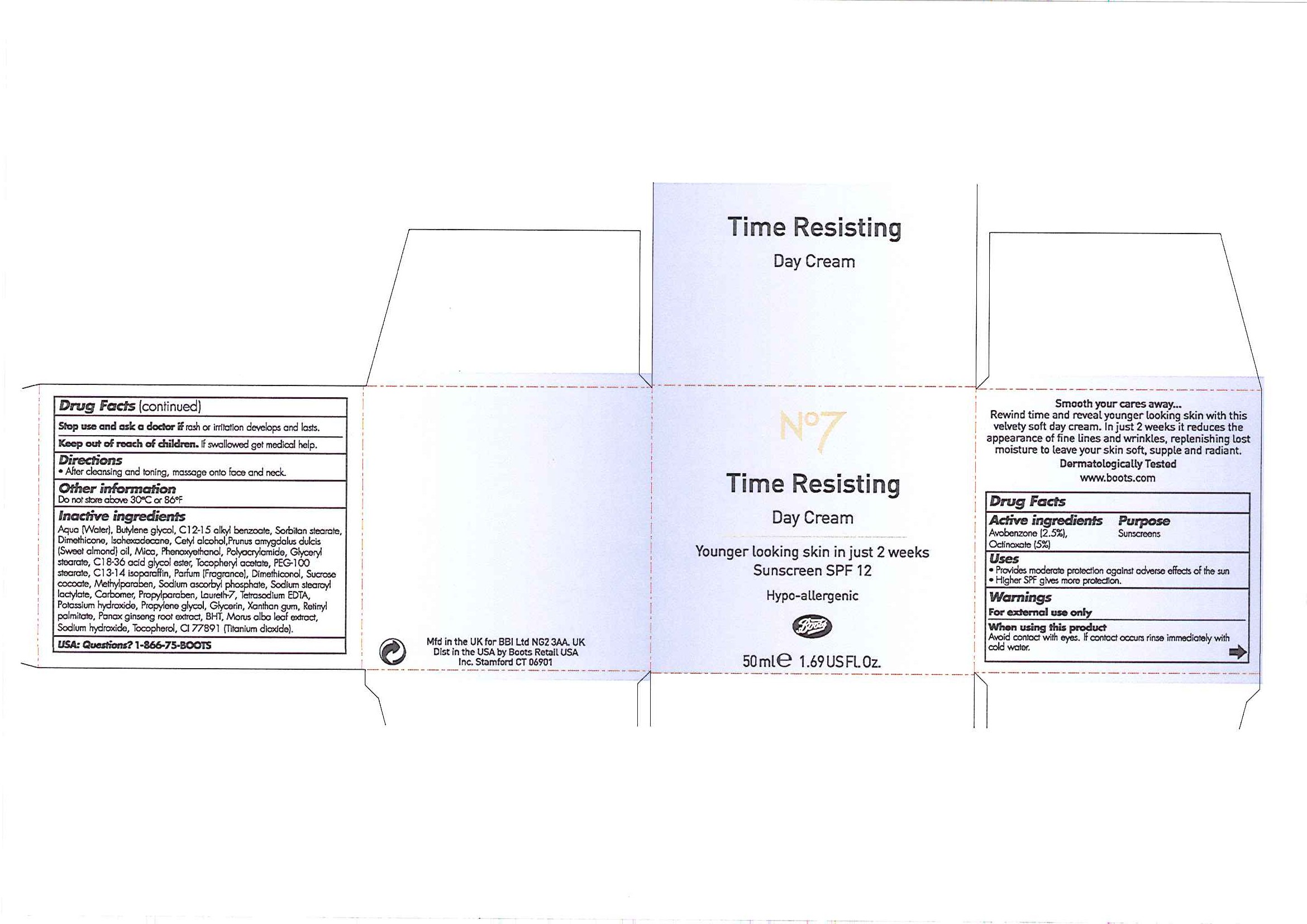
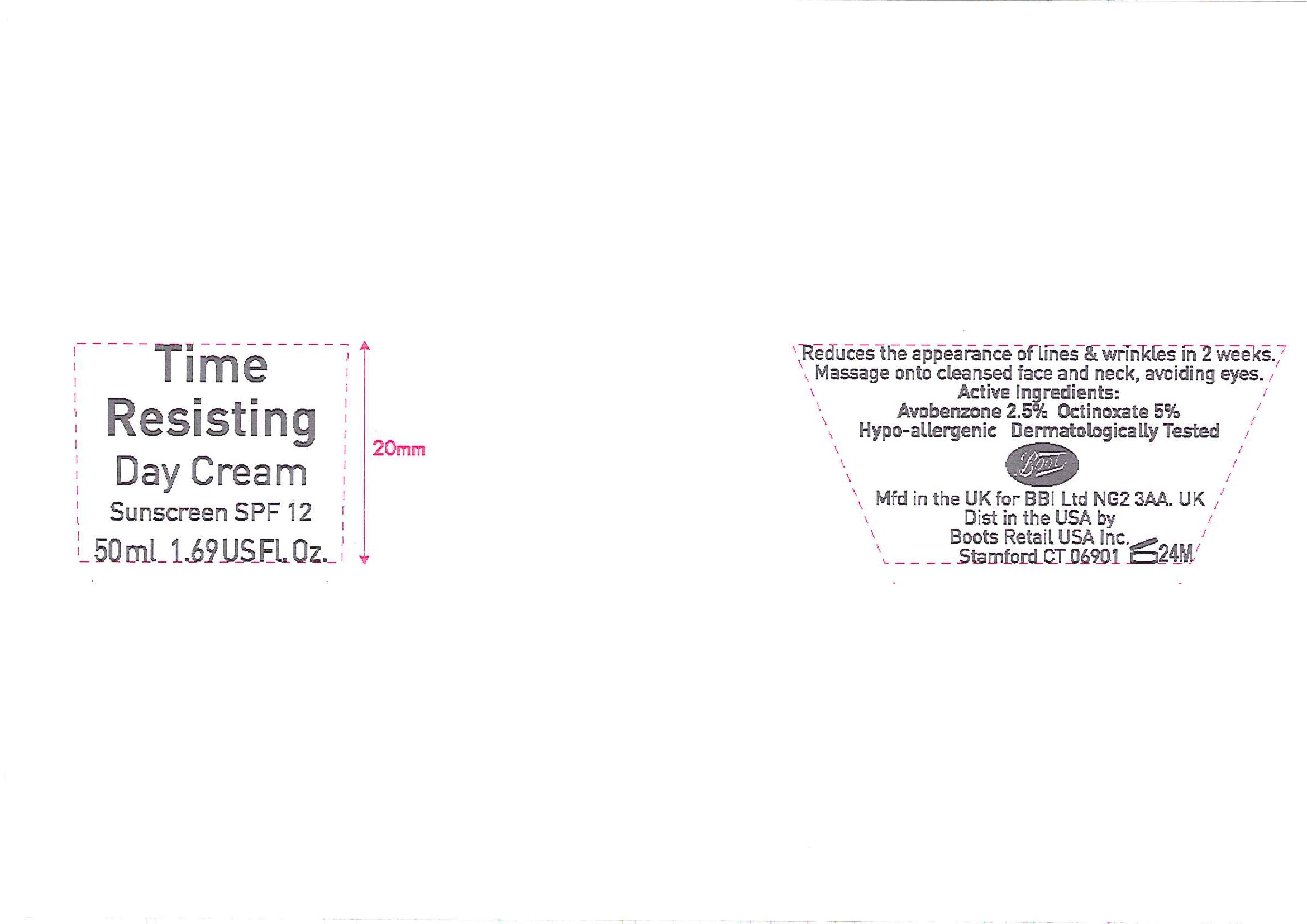
No7 Time Resisting Day Cream Sunscreen SPF 12
Smooth your cares away ...
Rewind time and reveal younger looking skin with this velvety soft day cream. In just 2 weeks it reduces the appearance of fine lines and wrinkles, replenishing lost moisture to leave your skin soft, supple and radiant.
Dermatologically Tested
www.boots.com
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS AND PRECAUTIONS
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients
Aqua (Water), Butylene glycol, C12-15 alkyl benzoate, Sorbitan stearate, Dimethicone, Isohexadecane, Cetyl alcohol, Prunus amygdalus dulcis (Sweet almond oil), Mica, Phenoxyethanol, Polyacrylamide, Glyceryl stearate, C18-36 acid glycol ester, Tocopheryl acetate, PEG-100 stearate, C13-14 isoparaffin, Parfum (Fragrance), Dimethiconol, Sucrose cocoate, Methylparaben, Sodium ascorbyl phosphate, Sodium stearoyl lactylate, Carbomer, Propylparaben, Laureth-7, Tetrasodium EDTA, Potassium hydroxide, Propylene glycol, Glycerin, Xanthan gum, Retinyl palmitate, Panax ginseng root extract, BHT, Morus alba leaf extract, Sodium hydroxide, Tocopherol, CI 77891 (Titanium dixoide).
- QUESTIONS
- INFORMATION FOR PATIENTS
- DESCRIPTION
- ACTIVE INGREDIENT
- INFORMATION FOR PATIENTS
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NO7 TIME RESISTING DAY SUNSCREEN SPF 12
octinoxate and avobenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11489-062 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.5 mL in 50 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1.25 mL in 50 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) DIMETHICONE (UNII: 92RU3N3Y1O) CETYL ALCOHOL (UNII: 936JST6JCN) ALMOND OIL (UNII: 66YXD4DKO9) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) POLYOXYL 100 STEARATE (UNII: YD01N1999R) METHYLPARABEN (UNII: A2I8C7HI9T) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) PROPYLPARABEN (UNII: Z8IX2SC1OH) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ASIAN GINSENG (UNII: CUQ3A77YXI) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SODIUM HYDROXIDE (UNII: 55X04QC32I) ALPHA-TOCOPHEROL (UNII: H4N855PNZ1) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11489-062-01 1 in 1 CARTON 1 50 mL in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/08/2009 Labeler - BCM Ltd (230780322) Registrant - The Boots Company PLC (218622660) Establishment Name Address ID/FEI Business Operations BCM Ltd 230780322 manufacture, analysis