Label: MAJOR REGULAR STRENGTH ASSORTED FLAVORS- antacid tablets tablet, chewable
- NDC Code(s): 0904-6412-92
- Packager: Major Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
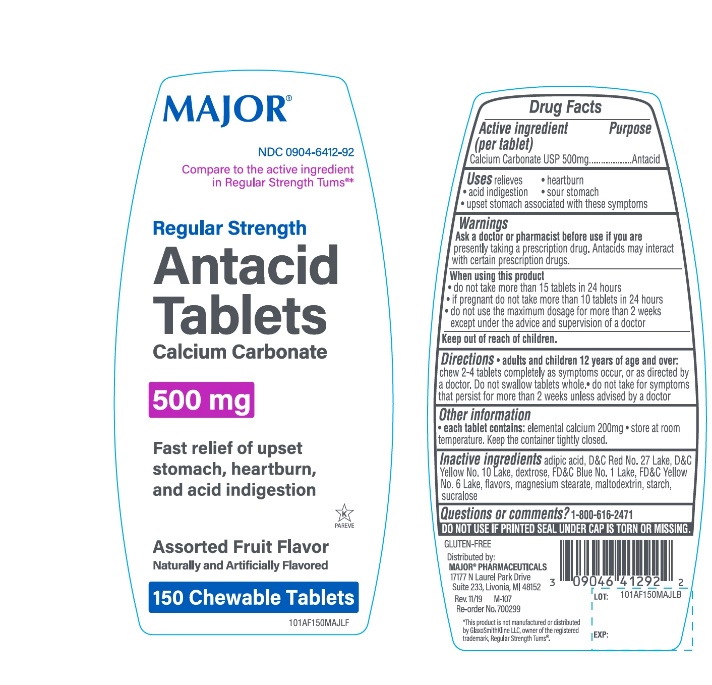
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor or pharmacist before use if you are presently taking a prescription drug. Antacids may interact with certain prescription drugs.
When using this product
- •
- do not take more than 15 tablets in 24 hours
- •
- If pregnant do not take more than 10 tablets in 24 hours
- •
- do not use the maximum dosage for more than 2 weeks except under the advice and supervision of a doctor.
- Keep out of Reach of Children
- Directions
- Other Information
- Inactive ingredients
-
Package/Label Principal Display Panel
Compare to active ingredient in Regular Strength Tums®*
MAJOR®
NDC 0904-6412-92
Regular Strength
Antacid Tablets
Calcium Carbonate 500mg
Fast relief of upset stomach, heartburn, and acid indigestion
Assorted Fruit Flavor
Naturally & Artificially Flavored
150 Chewable Tablets
K PAREVE
GLUTEN- FREE
Distributed by: MAJOR® PHARMACEUTICALS
17177 N Laurel Park Drive, Suite 233
Livonia, MI 48152, USA
M-107 REV. 11/19
*This product is not manufactured or distributed by GlaxoSmithKline LLC, owner of the registered trademark, Regular Strength Tums®.
-
INGREDIENTS AND APPEARANCE
MAJOR REGULAR STRENGTH ASSORTED FLAVORS
antacid tablets tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-6412 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 500 mg Inactive Ingredients Ingredient Name Strength ADIPIC ACID (UNII: 76A0JE0FKJ) D&C RED NO. 27 (UNII: 2LRS185U6K) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) STARCH, CORN (UNII: O8232NY3SJ) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color WHITE Score no score Shape ROUND Size 16mm Flavor FRUIT Imprint Code RP101 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-6412-92 150 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/12/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 08/12/2014 Labeler - Major Pharmaceuticals (191427277)